Nuclear Magnetic Resonance (NMR), a scientific method involving superconducting magnets at extremely low temperatures, is one of the main tools in the chemist's hands, and is used today for analytical tests ranging from metals and proteins to quantum computing research. In hospitals, a similar device, MRI, is used to diagnose a wide spectrum of medical conditions.
It may sound like magic, but two groups of scientists from laboratories at the University of Berkeley have now demonstrated that it is possible to perform chemical analysis by TMG without the use of magnets at all.
The TMD and MRI methods are based on the fact that many of the nuclei of the atomic elements have spin (a quantum property) and similar to the north and south magnetic poles of the Earth - they have bipolar magnetic fields. In the normal NMG method, these nuclei line up in the same direction when exposed to an external magnetic field, then change their direction when exposed to radio waves. The rate at which each type of nucleus "oscillates" as a result is unique and allows identification of the element; For example - a nucleus of hydrogen-1, a single proton, oscillates four times faster than a nucleus of carbon-13, which contains six protons and seven neutrons.
The ability to detect these signals depends first and foremost on the ability to locate the spin itself; If in the example there is an identical number of nuclei with up-spin and nuclei with down-spin, the total polarization will be zero, and the signals will cancel each other out. However, since spin-up directionality requires slightly less energy, the population of atomic nuclei has little excess spin-up.
"The existing scientific view claims that trying to perform NMG measurements using zero or weak magnetic fields is a bad idea, since the polarization is tiny, and the ability to detect signals is characteristic of the strength of the applied external field."
"GDP using zero or weak magnetic fields starts with three characteristics working against it: small polarization, low detection capability and the absence of a chemical shift signature," explains the lead researcher. "Then why make an effort for that?" asks one of the researchers. "The big advantage is the ability to get rid of the large and expensive magnets needed in a normal GDP. If we manage to do this, it will be possible to develop a portable NMG device and reduce its operating costs. Our hope is that there will be the ability to conduct chemical analysis in the field - underwater, in boreholes and in ballooning balloons - and perhaps even make medical diagnoses far away from advanced medical centers."
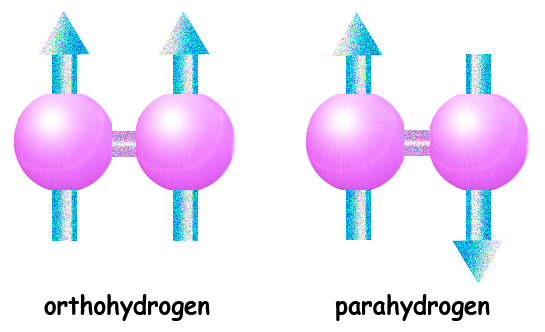
"It turns out that there are already methods that overcome small polarization and low detection ability," explains the lead researcher. The directionality of the spin can be increased in several ways, collectively called "over-polarization". One of the ways to superpolarize a hydrogen gas sample is to change the ratio between para-hydrogen and ortho-hydrogen found in it. Like most gases, at normal temperature and pressure each hydrogen molecule contains two atoms bonded together. If the spins of the proton nuclei are oriented in the same direction, this type of particle is called ortho-hydrogen. If the spins point in opposite directions, the particle is called a para-hydrogen. The spin states of two protons and two electrons in a hydrogen molecule lead to three ways in which ortho-hydrogen can attain a spin equal to 1; A para-hydrogen can only have spin equal to zero. Ortho-hydrogen molecules normally make up three quarters of the hydrogen gas and the rest para-hydrogen. The amount of para-hydrogen can be increased to fifty percent or even a hundred at very low temperatures, although the appropriate catalyst must be added, or else the conversion may take days if not weeks. With this method it is possible to obtain highly polarized hydrogen gas.
For weak magnetic fields, increasing the detection capability requires a completely different approach - the use of detectors called magnetometers. Despite the fact that these detectors are extremely sensitive, they still need to be cooled to very low temperatures. Optical-atom magnetometers measure the whole atom, not just the nucleus. In this method, an external magnetic field is measured by measuring the spin of the atoms inside a vapor chamber of the magnetometer, usually a dilute gas of an alkali metal such as potassium or rubidium. Their spin is affected by the polarization of the atoms using a laser beam; If there is an external field, even a weak one, they start to oscillate. A second laser beam measures the rate of oscillations and thus the strength of the external field can be determined. The research group brought this method to a high level by increasing the "relaxation time" - the time it takes for the polarized gas to lose its polarity.
"No matter how sensitive your detector is or how polarized your sample is - chemical sets cannot be measured in a field equal to zero," explains the researcher. "However, there was always another signal in GDP that could be used for chemical analysis - it's just that it's usually much weaker compared to chemical shifts, and it's called J-coupling." This coupling refers to the interaction between two protons (or any two other nuclei with spin), mediated by their electrons. The characteristic frequencies of these interactions, which appear in the NMR spectrum, can be used to determine the angle between the chemical bonds and the distances between the nuclei. The signals obtained from this coupling are highly specific and able to indicate the nature of the measured chemical composition.
At this stage, the researchers built a unique magnetometer built specifically to detect J-coupling in a magnetic field equal to zero. "The first step is to introduce the para-hydrogen sample," explains the researcher. "The upper part of the system is a test tube containing the sample solution into which the para-hydrogen is bubbled." Below the test tube is the alkaline vapor chamber of the magnetometer, the size of which is smaller than a fingernail. The cell contains rubidium and hydrogen gas and is surrounded by a cylinder made of a nickel-iron alloy that functions as a shield against external magnetic fields.
Although all the experiments conducted to date have been carried out with easily hydrogenable molecules, the method can also be extended to other types of molecules. Explains the main researcher: "We are only at the beginning of the development of a GNP in a field equal to zero, and it is still too early to say if we can compete with a GNP with a high field." However, we have already demonstrated that it is possible to obtain a very clear and specific spectrum using a device that allows for portable and cheap chemical analysis."

4 תגובות
I also did not understand why the person who wrote the article wrote that for maximal polarization the amount of para-hydrogen should be increased - after all, the para-hydrogen is zero - the amount of ortho-hydrogen should be increased in order to create more signal.
In this sentence:
The spin states of two protons and two electrons in a hydrogen molecule lead to three ways in which ortho-hydrogen can attain a spin equal to 1; A para-hydrogen can only have spin equal to zero…
Why does ortho-hydrogen have 3 ways to reach a spin equal to 1? (I understand that the para-hydrogen resets itself).
So the sample needs to be treated first?
Amazing! Successfully