Scientists from the Carnegie Institution have discovered for the first time that high pressure can be used to make a unique material for storing hydrogen. The discovery paves the way for a completely new possibility to deal with the issue of hydrogen storage
Scientists from the Carnegie Institution have discovered for the first time that high pressure can be used to make a unique material for storing hydrogen. The discovery paves the way for a completely new possibility to deal with the issue of hydrogen storage.
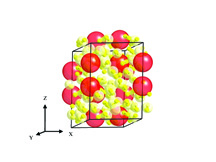
The researchers discovered that the inactive noble gas xenon binds to molecular hydrogen (H2) under pressure to form a new solid with unusual bonding chemistry. These experiments are the first in which these elements reacted together to form a stable compound. The discovery introduces for the first time a new family of materials, which could grow innovative hydrogen technologies. An article about the research was published in the scientific journal Nature Chemistry.
The element xenon has several interesting properties, including its use as an anesthetic, its ability to preserve biological tissues and its use in lighting fixtures. Xenon is a noble gas, and therefore it does not react with other substances or elements under normal conditions, usually.
The lead researcher, Maddury Somayazulu, from the Geophysics Laboratory at the Carnegie Institution, explains: "Elements change their electronic arrangement under pressure, similar to how passengers adjust their position when an elevator is filling up. We reacted series of gaseous mixtures of xenon with hydrogen at high pressure inside a diamond chamber. At a pressure that is forty-one thousand times the pressure at sea level (one atmosphere) the atoms begin to arrange themselves in a lattice structure (a net-like structure) in which the hydrogen atoms are the majority of the components with layers of weakly bound xenon pairs. When we increase the pressure, similar to radio tuning, the distance between the xenon pairs changes - the distances shorten and become those observed in compressed metallic xenon."
The researchers tested the compound at varying pressures using advanced measurement methods such as X-ray diffraction and Raman and infrared spectroscopy. When they looked at the xenon part of the structure, they realized that the interaction between the xenon and the hydrogen surrounding it is responsible for the unusual stability and the continuous change in the distances between the xenon atoms when the pressure reached XNUMX atmospheres.
Why was the compound so stable? "We were completely surprised by both the structure and the stability of this material," explains one of the researchers. Once the electron density is stretched toward the nearby hydrogen atoms, this appears to stabilize the compound and the xenon pairs.
"Xenon is too heavy and expensive to be practical for use in hydrogen storage applications," explains the lead researcher. "However, by understanding the mechanism in which the phenomenon occurs, researchers will be able to find lighter and more practical materials."
"It is very exciting to find new high-hydrogen compounds, not only for our interest in simple molecular systems, but because such discoveries can form the basis of important new technologies," explains one of the researchers. "This high-hydrogen solid represents a new way to prepare innovative hydrogen storage compounds and the new pressure-based chemistry paves the way for the synthesis of novel high-energy materials."
The news from the research institute

One response
Can you give an example of the practical benefits of this discovery?
I guess that doesn't mean that this substance is stable in a normal atmosphere, that is, to the best of my basic understanding of chemistry, as soon as you lower the pressure, the compound breaks down.
So the question is, why is it practical?