One of the solutions to the gap between the ability of alternative energy systems to produce energy and the times and places of consumption is to store excess energy in electrolytic cells and use them during hours when not enough energy is produced
The need to reduce carbon dioxide emissions requires the use of a higher proportion of renewable energy from the total energy. This conclusion accelerates the development of new solutions that require significant changes in the percentages of the use of wind energy, hydrological energy and solar energy, etc. One of the solutions is a distributed energy system. In this system it will be possible to store excess energy locally using, for example, local electrolytic cells.
Today, most of the energy is produced by regional power plants based on fossil fuels such as: coal, oil and natural gas. In addition, there is the energy produced by hydroelectric power plants, nuclear power plants and wind farms. This energy flows in one direction only - from the station to the electricity grids and from there to the consumers. Today, the idea is accepted that this system should be provided with a much larger amount of energy coming from renewable sources. One of the possibilities to realize this lies in a distributed energy system.
A distributed energy system consists of a large number of small production units spread over a wide geographical area and a small number of larger central units. The separate parts of the energy distribution system operate independently, but can also be integrated together through the use of information technology (IT, a general name for computing methods, information processing and data communication), thereby allowing the use of both the local output and the central output to supply the full need for that moment.
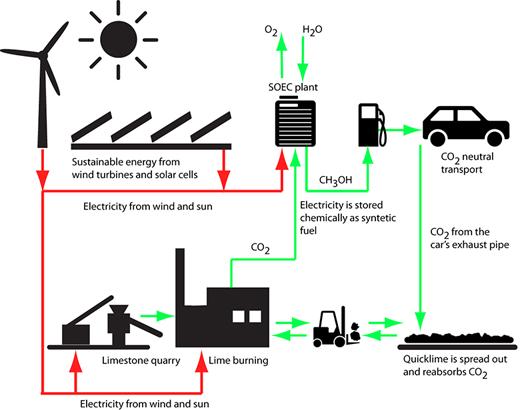
Such a system will require the development of an ability to convert excess electricity from renewable sources into energy that can be stored and used when needed. One of the options is to store the excess output as chemical energy, for example in the form of compounds such as liquid methanol or gases (such as natural gas) or gas - carbon monoxide and hydrogen. Once the energy has been converted into these chemical compounds, known as synthetic fuels, they are much easier to store in tanks. The synthetic fuels could be used directly in vehicles and as starting materials for the chemical industry. In fact, there is nothing new in this idea. The only problem is that the technologies that exist today are adapted to large central plants that use high temperatures. Therefore, it is necessary to develop new types of plants that use lower temperatures and are suitable for their combination with local wind turbines.
The conversion of electricity into chemical energy requires the use of electrolytic processes. Through electrolysis, water is converted into hydrogen and oxygen by an electric current. "For this purpose, electrolytic cells of the SOEC type are being developed today," explains Professor Mogens Mogensen from the University in Denmark. "This type of electrolytic cell consists of ceramic materials."
The process that occurs in electrolytic cells is actually parallel to the stage in nature's photosynthesis, where green plants absorb carbon dioxide from the air and convert it into chemical energy that is stored as sugars. Therefore, electrolytic cells may contribute to the removal of carbon dioxide from the air. In other words, these cells are similar to the role of forests of trees in absorbing carbon dioxide from the air.
By converting carbon dioxide into liquid synthetic fuels in an electrolytic cell, our means of transportation will be able to consume sustainable energy, energy from wind turbines and solar cells. When a car uses synthetic fuel, it does indeed emit carbon dioxide into the air, but it is in the same amount that was used to produce the synthetic fuel in the first place - that is, in the bottom line, no amount of carbon dioxide was added to the atmosphere.
Cells operating at high temperatures are more efficient compared to other electrolytic methods because they produce more oxygen and carbon monoxide from the same amount of electricity. This result is obtained because at high temperatures the use of heat causes the decomposition of water and carbon dioxide into gases (hydrogen and carbon monoxide) and oxygen, and therefore this type of cell cools itself: the heat that is inevitably generated when electricity flows through a certain component is used to promote the electrolytic process himself. In addition, the excess heat obtained in chemical processes occurring in industry or factories can be used for this very purpose.
"These electrolytic cells will be very suitable for central and large factories for the production of synthetic fuel from gas. The catalytic stages that follow the electrolytic process require the existence of an overall facility with a catalytic reactor connected to a system of electrolytic cells since the synthetic hydrocarbons obtained at this stage are not stable at such a high temperature (over 650 degrees Celsius). "Such a facility would probably have to exceed 100 million watts to be economically viable," says the researcher. In addition, heat loss is avoided in large factories. For local production conditions, it is necessary to develop cells that can operate in a temperature range of between two hundred and four hundred degrees Celsius. In this way, it will be possible to establish local and small electrolytic plants that can be connected directly to local wind turbines and produce synthetic fuel for local use. "Our vision is that we will be able to build small and modular factories, each of which will be connected to a local wind turbine," explains the researcher.
Low temperatures mean less heat loss and allow easier construction of small, modular electrolytic plants. For the success of this project, it is necessary to develop completely new materials. In the current project, we are testing two types of electrolytic cells - one consists of a mesoporous ceramic material, which is capable of adsorbing liquid electrolytes to the nanopores and keeping them inside. The second type is based on low-temperature proton-conducting materials containing a solid ceramic electrolyte.
Separating carbon dioxide directly from the air is complicated and expensive. The main researcher recognizes in his mind's eye, because of this, the need to absorb the gas from other sources. For example, breweries and alcohol factories, where large amounts of gas are obtained in the fermentation processes. Another option lies in the use of the most common raw material in Denmark - limestone (calcium carbonate). Heating this material releases carbon dioxide and the material calcium oxide is obtained. Normal water is added to this material and then calcium hydroxide is obtained (slaked lime, pulverized quicklime used as a mixture for plastering walls as well as for agriculture) so that most of the heat introduced is released outside, that is, this method maintains a constant concentration of the gas without releasing it into the open air.

One response
Finally a smart idea for the production of synthetic fuels that do not depend on fossil fuels, the question is whether it is economic? And perhaps for governments to subsidize such enterprises.