A global breakthrough - Technion researchers have demonstrated the possibility of dispersing tiny carbon tubes in "superacid" * For the first time it has been shown that this can be done as a first step in spinning nanowires from tiny carbon tubes
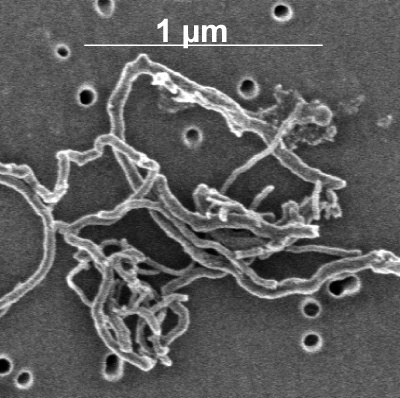
The Technion researchers were able to prove for the first time that there is a possibility of dispersing tiny carbon tubes in "superacid". This is a breakthrough that will lead to revolutionary developments in material science and nanoelectronics, and may be the first step in spinning nanowires from tiny carbon tubes. This is reported in an article published in the prestigious scientific journal "Nature Nanotechnology".
"One of the most interesting materials in nanotechnology are tiny tubes of carbon," explains Professor Yeshayahu Talmon from the Technion's Faculty of Chemical Engineering. "The challenge facing researchers around the world is - how to turn such a tube, which has a diameter of 1-2 nanometers (one billionth of a meter), into something useful on a large scale, such as efficient cables for electrical transmission or strong and light construction materials. Because in production, these strong and tiny tubes arrive when they are mixed, a sort of 'spaghetti', and the challenge is to disperse and separate them into individual tubes, and then weave them into threads in which the tubes are well arranged."
A group of researchers from "Rice" University in Houston, Texas, led by Professor Matteo Pasquali, the colleagues of the Israeli researchers, Professor Isaiah Talmon and Professor Yachin Cohen, proposed to sprinkle the tiny tubes with chlorosulfonic acid ("super-acid"). It is a very strong acid, which can seriously damage people and equipment if used carelessly. It must also be ensured that water vapor from the air does not come into contact with it. The challenge facing the Technion researchers was to prepare samples of this acid and put them in an electron microscope. Following a development process, they were able to safely prepare models that preserved the structure of the tubules in the acid. "We were able to develop a method in which we produced such models, for low-temperature penetrating electron microscopy (cryo-SEM), and thus we showed that indeed the tubes are nicely dispersed in the acid," says Professor Talmon. "This is the first time it has been shown that these nanotubes can be dissolved in this superacid. Moreover, it has been proven that there is a good chance of weaving threads based on tiny carbon tubes."
"Professor Talmon and his colleagues at the Technion made a vital contribution required to prove that nanotubes spontaneously dissolve in chlorosulfonic acid," said Professor Pasquali. "For this they had to develop new experimental methods."
Scientists hope that in the future they will be able to use the fibers spun from tiny carbon tubes to efficiently conduct electricity over long distances and to create building materials that are very strong and lighter than conventional materials.

6 תגובות
Congratulations to Ishii and Lichhan for the achievement.
------
Enter my blog - Another science
And of course congratulations to Yishai and Yachin for the discovery. They really deserve it
"In production, these strong and tiny tubes arrive when they are mixed, a kind of 'spaghetti', and the challenge is to disperse and separate them into individual tubes, and then weave them into threads in which the tubes are well arranged."
Think of the fact that you put spaghetti in water and then you can separate the spaghetti one by one. That's the idea
and my people,
A super strong acid, it seems to me that its ph is just really low. 0.5 or maybe less.
light:
You will read "... models that preserved the structure of the tubules in acid".
The tubes were not dissolved in the sense of breaking their internal structure, but in the sense that they exist in liquid. Therefore, it is possible to transport them using a liquid, which is significant for industrial processes.
What makes chlorosulfonic acid a "superacid"? What does the phrase mean? Are sulfuric acid or hydrochloric acid not super acids?
It is not clear how from the fact that the acid dissolved the nanotubes they concluded that it is possible to grow nanotubes in the best case they will get graphite