Unlike hydrocarbon-based fuels, which emit greenhouse gases and other toxic pollutants, the only combustion product of hydrogen is water. compared to gasoline
Since the seventies, hydrogen has been treated as a promising alternative to fossil fuels thanks to its clean combustion - unlike hydrocarbon-based fuels, which emit greenhouse gases and other toxic pollutants, the only combustion product of hydrogen is water. Compared to gasoline, hydrogen is light-weight, able to provide higher energy density and is particularly available. However, there is a reason why we still do not enjoy a hydrogen economy: in order to replace gasoline as a fuel, hydrogen must be stored safely and compressed, and at the same time be easily accessible. Given the limited availability of materials capable of jumping over these conflicting bumps, hydrogen storage technologies lag behind other clean energy supply alternatives.
In recent years, researchers have been trying to attack these two obstacles by trapping hydrogen inside solids while storing large amounts in small volumes with low chemical activity - an essential characteristic for keeping this volatile gas in a stable state. However, most of these types of solids are able to absorb a relatively small amount of hydrogen and require extreme heating/cooling in order to generate sufficient energy efficiency.
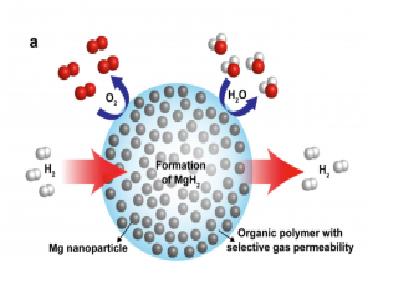
Now, scientists from the Lawrence Berkeley National Laboratory of the US Department of Energy have developed an innovative composite material designed to store hydrogen and which consists of metallic magnesium nanoparticles dispersed within a substrate of polymethyl methacrylate, the polymer at the base of the common material Plexiglas (plastic glass). This nanocomposite material absorbs and emits hydrogen quickly at moderate temperatures without oxidizing the metal after recycling - an ability that is a significant breakthrough in the design of materials intended for hydrogen storage, and for the development of batteries and fuel cells.
"This research showcases our ability to design composite nanomaterials that are able to overcome fundamental kinetic and thermodynamic barriers and help obtain materials that have so far been extremely elusive," says Jeff Urban, one of the lab's directors. "Furthermore, we are able to leverage the unique properties of both the polymer and the nanoparticle found in this composite to solve similar problems in other areas of energy research."
The researchers used a microscope to observe the individual magnesium nanocrystals dispersed within the polymer. Using the high-resolution imaging capabilities of the world's most powerful electron microscope in this lab, the researchers were also able to locate defects—atomic voids in a seemingly perfect, ordered crystalline system—while gaining unprecedented insight into the behavior of hydrogen within this new family of storage materials.
"Discovering new materials capable of helping us develop sustainable energy solutions is at the core of the Ministry of Energy's purpose. Our laboratory provides unprecedented experiments to support this purpose with great success," says the researcher. The research findings were published in the scientific journal Nature Materials.

One response
Finally, the magnesium of the Dead Sea will become a required resource!!!!….. The Levies….