On the discovery of bucky balls - a molecule containing 60 carbon atoms arranged exactly like a football
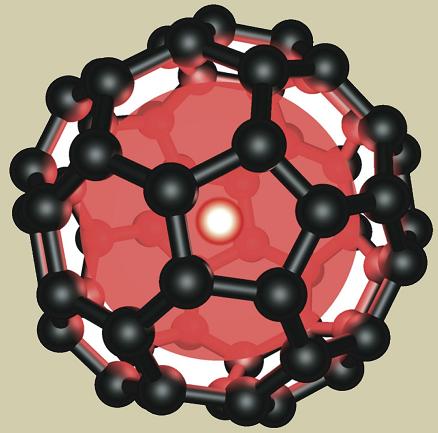
"Guys, what you have is just football." This is what the mathematician William Veitch told the chemists Harry Croteau (from the University of Sussex, England) and Richard Smoley (from Rice University, Texas, USA). He meant the geodesic physical structure of a new molecule of pure carbon, containing 60 carbon atoms, which the two presented to him.
In fact, the existence of this giant molecule was predicted several years before its actual discovery. David Jones, who wrote a column about imaginary inventions in the New Scientist weekly (under the pseudonym "Daedalus"), previously wrote that the carbon atoms can arrange themselves in a lattice of hexagons and pentagons, which is shaped like a hollow sphere. The physical structure of the new molecule is indeed very reminiscent of the structure described by "Daedalus", and it is the same as the structure designed and built by the architect Beckminster Fuller at the international exhibition "Expo 67", which took place in Montreal, Canada, and was called a "geodesic dome".
How is a diamond made?
The story of the discovery of the "molecular foot ball" began when Hari Croteau used spectroscopic methods to study clusters of carbon atoms, such as graphite or diamond, and other chains of carbon found in interstellar space.
Smoley built a new type of laser, which enabled the investigation of small clusters of atoms. Using this laser, the surface of a solid crystal containing atoms of some element is vaporized. The resulting vapors are pushed by means of a jet of inert gas into space, where the atoms combine into clusters. Smoley was at the time engaged in atomic research of Zoran.
Croteau realized that by means of the system built by Smoley it would be possible to reproduce the conditions existing on the surface of giant stars emitting vaporized carbon. The two, who met through a mutual friend, decided to work together.
Supersonic jets of helium
They vaporized carbon using a powerful laser beam, and transported the vapor, using a supersonic jet of helium, into a chamber where vacuum conditions prevailed. In this room the molecules were ionized) charged with an electric charge. (Using a mass spectrometer, the atomic weight of individual carbon gas particles was examined.
One of the researchers in Smalley's research group, Harry Heath, noticed in the spectrometer diagram, peaks indicating molecules containing 60 carbon atoms, which appeared relatively frequently. Using different techniques, the researchers were able to increase the prevalence of these molecules even more.
The free ends of "Flatland"
At this stage, Smoley believed that graphite-like surfaces of carbon were formed there, which stick together. Kroto proposed a flat model with curved edges called "Flatland". But these models had a notable drawback: they had to have free ends that could react chemically with other materials. But the molecules that contained 60 carbon atoms were very stable, and did not react with any substance. Even benzene, which is a closed cyclic compound, reacts with all kinds of compounds because of the hydrogen atoms at its vertices. But the new carbon A contained hydrogen atoms. The meaning of the "haughty" behavior of the new molecule is that it is built in a closed structure, without free or free ends.
.
This is how the team arrived at the possibility of a geodesic structure. Croto recalls that he once built a model of a dome made of cardboard for his children at home. He vaguely remembered that it was made of hexagons and pentagons, but he wasn't sure of that, and went to look for the toy dome in the children's room. In the meantime, Smalley obtained Robert Marx's book "The Dynamic World of Buckminster Fuller" in which appeared, among other things, a photograph of the Giadez dome that Fuller built at the time in Baton Rose (*check the name of the place) for a car company called "The Union of Tanker Manufacturers." But he did not Noticed that this structure was built of pentagonal and hexagonal planes.
.
A hard day's night
.
At the end of that grueling day, Harry Heath went home, and there he tried, together with his wife, to build a model of the desired molecule from toffee candies and matches. He did not succeed. That evening Smolay was also sitting in his house, trying to build a model out of small cardboard cubes. When he tried to stick them together to get a spherical shape, he realized that after a few rows that created a sort of "salad bowl", the structure reached a dead end. At midnight, Smoley remembered Croto's hint about the pentacles.
He cut suitable pentagons and began to connect them to the "salad bowl". "Then he found that the pentagons connect nicely with the hexagons and that's how he got a hemisphere built from 12 pentagons and 20 hexagons. From here to the completion of a complete cadok, containing 60 vertices, the distance was short. At each vertex in the structure there was a meeting of a pentagon and a hexagon - which symbolized one carbon atom.
The next day, after presenting the model in the laboratory, Smoley called Weitz, 'so that he could examine the feasibility of the structure from a mathematical point of view. In response, Vitch said what he said about the football - but his tests showed that the structure was indeed possible. Instead, the new molecules were named "Becky balls" after the architect Buckminster Fuller, that the new molecules are built, as it were, according to the model of the geodesic dome he designed. Later in the research, it was found that it is possible to capture in the new "carbon balls", various metal atoms, whose physical size is suitable for this (for example, lanthanum).
A sweltering flame
In the meantime, Klaus Hoffmann from the Institute of Physics in Darmstadt found that already in 1978 it was known that in a smoldering flame, the molecules containing 60 carbon atoms appear, but when they reach the blue (oxidizing) part of the flame, they burn. the structure of "Bucky balls," through a nuclear magnetic resonance examination.
In the meantime, some American and German researchers found a way to produce the "Bucky balls" in quantities of milligrams per day, while Smolay and his students already know how to produce several tens of grams per day at a price that does not exceed that of aluminum production.
.
What do we get out of this?
.
The possibilities of practical uses for the new carbon molecules are many. Due to its great stability, the material can be used as a perfect lubricant, or, even, as a kind of molecular bearings. It can form the basis of new types of organic compounds with possibilities of use in medicine, efficient electrochemical cells, a basis for the development of new batteries, and even research into the ways in which various substances are formed in the stars and in interstellar space.
Epilogue
Smoley and Croteau won the Nobel Prize in Chemistry for their discovery in 1996. Smoley passed away in 2005. Croteau assists in raising funds for scientific research, among other things by means of a "dinner raffle with the Nobel laureate Hari Croteau".
The article appeared on the blog of Bischem Azgad

3 תגובות
As someone who taught towards the end of a very long chapter of technology and science teaching also the subject of fullerene, the presentation of the unique structure of the molecule as the same structure as football is an outstanding presentation, both in terms of illustration and in terms of bringing a "scary scientific" subject to a popular and much more understandable field. Well done !
Nano bearings in nano motors. Science fiction that may come true just around the corner...