A mesh that includes a nanometer coating is able to trap oil while allowing water to pass through it without any restriction. Further development of this technology could lead to the absorption of oil spills with the help of a large network
[Translation by Dr. Nachmani Moshe]
A mesh that includes a nanometer coating is able to trap oil while allowing water to pass through it without any restriction. Further development of this technology could lead to the absorption of oil spills with the help of a large network.
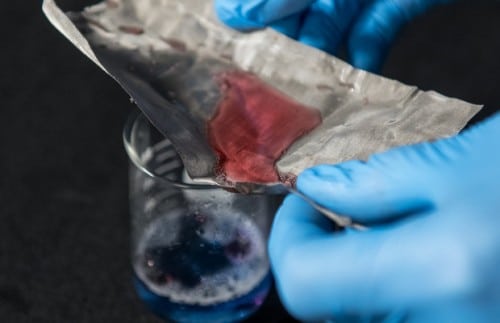
A humble piece of stainless steel mesh in a research lab at Ohio State University doesn't look like much, but it could make a big difference in environmental cleanup—water passes easily through this mesh while oil doesn't, thanks to an almost invisible oil-repellent coating on on the surface of the network.
In their experiments, the researchers mixed water and oil and poured this mixture through the mesh. The water passes through the net and is collected in a container. The oil is collected on top of the net and is easily poured into a separate container by tilting the net in its direction. The mesh's coating is part of a collection of nanotechnologies developed inspired by nature at Ohio State University and detailed in the journal Nature Scientific Reports. The possible applications of the new technology range from cleaning up oil spills to locating underground oil deposits. "If we scale up our lab piece, we can capture leaked oil with a net," said Bharat Bhushan, professor of mechanical engineering at Ohio State University and the lead researcher.
The study was inspired by the leaves of the lotus plant, whose rough surface naturally repels water, but not oily substances. In order to create the coating with the opposite ability, i.e. - repelling oiliness - the researchers chose to coat a bumpy surface with a polymer that includes molecules of a surface-active substance (surfactant) - the same substance that gives cleaning agents their ability to repel oiliness. The researchers sprayed a fine powder of silica nanoparticles onto the stainless steel mesh to create a bumpy surface and then added the polymer layer on top. All four components: the silica, the surfactant, the polymer and the stainless steel are non-toxic and inexpensive, explains the lead researcher. Therefore, he estimates that it is possible to produce a large network of this type that will cost less than one dollar per 0.1 square meter.
In light of the fact that the coating is only hundreds of nanometers thick (a billionth of a meter) it is almost invisible. When touched, the mesh does not feel bumpier than an uncoated mesh. The mesh with the coating is less shiny, this is in light of the fact that the coating itself is only 70% transparent. The researchers chose silica because this material is a component of glass and they wanted to test the potential of this technology for developing a glass coating that repels oily stains. The researchers found that at a transparency level of 70% the coating could be effective in certain glass applications in the automotive industry, such as mirrors, but not in most windows in homes and mobile phone displays. "Our goal is to achieve a coating with 90% transparency," explains the lead researcher. "For our coating, different combinations of the components in the different layers give rise to different properties. The trick is to choose the right combinations." The researcher explains that certain combinations of layers gave rise to nanoparticles that actually bound to the oil instead of rejecting it. Particles of this type could, for example, be used to locate underground oil or help remove it in case of oil leaks.
The shape of the nanoparticles is also important. One of the researchers on the team is looking at what will happen when the surface is composed of nanotubes instead of a mesh. And instead of using silica, they are looking at molybdenum disulfide nanotubes that mix well with oil and petroleum. The researchers examine the physical load that these tubes can withstand and their friction properties. They believe that such a material that will have reduced friction can be used as an effective additive in lubricants. They could also be used as lubricants themselves in micro- and nano-devices.
The research began more than a decade ago, when lead researcher Bhushan began researching and developing coatings with nanostructures that mimic the texture of lotus leaves. From this idea, he and his team of researchers continued to increase this capability and adapt it to different applications. "We tested so many natural surfaces, from leaves and butterfly wings to shark skin, in order to understand how nature solves various problems," says the lead researcher. "Now we are beyond the capacity of nature, this is in order to solve new problems."

One response
So