Scientists from the IBM company succeeded in obtaining an "anatomical" image - or a precise chemical structure - of a dissected face, an image with unprecedented resolution, through the use of a complex method known as "non-contact atomic force microscopy".
Scientists from the IBM company succeeded in obtaining an "anatomical" image - or a precise chemical structure - of a dissected face, an image with unprecedented resolution, through the use of a complex method known as "non-contact atomic force microscopy".
The findings could speed up research in the field of using particles and atoms on the smallest scale and have a significant impact on the field of nanotechnology, seeking understanding and control over the smallest components known to man.
"Although the comparison is not the same, if you imagine how a doctor uses X-rays to image bones and organs inside the human body, then we use an atomic force microscope to image atomic structures that are the skeleton of individual animals," explains researcher Gerhard Meyer.
"Mapping and scanning methods with the help of detectors offer great potential for preparing prototypes for complex functional structures and for designing and examining their electronic and chemical properties at the atomic level."
The current article by the research team follows another experiment published just two months ago in the prestigious journal Science, in which the IBM scientists measured the charge states of atoms using an AFM microscope. These breakthroughs will give rise to new possibilities for examining the ways in which electric charge passes through insulators or their networks. Knowing the distribution of the charge at the atomic level is essential for the preparation of tiny computing components, faster and more efficient than those existing today in the various processors and memory devices.
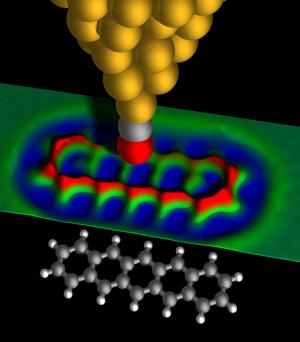
In the new study, published in the scientific journal Science on August XNUMX of this year, IBM researchers used an AFM microscope operating in an extremely high vacuum (very low pressures) and at very low temperatures (minus two hundred sixty-eight degrees Celsius) to image the chemical structure of pentacene compounds (pentacene, the value in Wikipedia) individuality. The scientists were able, for the first time ever, to "look" into the "electron cloud" and see the atomic skeleton of a single particle. Despite the existing differences, the method resembles X-rays that pass through soft tissues and allow a clear image of the bones.
The AFM microscope uses a very sharp metal tip to measure tiny forces between it and the material sample to obtain an atomic image. The compound tested in the current experiments was pentacene - an elongated organic compound consisting of twenty-two carbon atoms and fourteen hydrogen atoms that is 1.4 nanometers wide. The distance between neighboring carbon atoms is only 0.14 nanometers. In the resulting image, you can clearly see the hexagonal pattern of the inner carbon rings that make up the part. From the picture you can even deduce the exact location of the different hydrogens.
"The key to achieving such an atomic separation capability was an atomically defined and sharp tip as well as the great stability of the chosen system," explains one of the researchers. In order to get an image of the chemical structure of the slag with the help of an AFM microscope, it is necessary to operate as close as possible to the slag itself. The range in which the chemical interactions give rise to significant effects for the electrical forces is less than one nanometer. In order to achieve this goal, the researchers were required to increase the sensitivity of the microscope tip and overcome a significant limitation: similar to the situation where two magnets will repel or attract when they approach each other, the particles can easily be repelled or attracted to the tip when it comes too close to them - while preventing any further measurement .
The researcher adds, "We were able to prepare a unique tip that is able to "react" with atoms or single particles and thus obtain a very high separation capacity." A tip coated with carbon monoxide (CO) produced the best resolution when it was only half a nanometer away from the sample being examined - and used as a powerful magnifying glass - it showed the individual atoms in the pentacene sample while revealing its exact atomic structure.
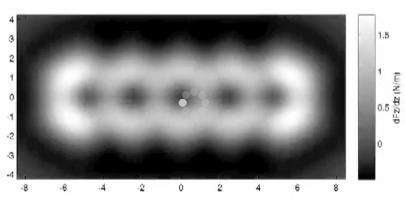
In addition, the scientists were able to produce a complete XNUMXD force map of the tested specimen. "In order to achieve this, the microscope is required to be extremely stable, both mechanically and thermally, to ensure that both the tip and the parts remain unchanged during the collection of information that lasts about twenty hours," notes one of the researchers. Further theoretical research also produced more in-depth explanations of the effectiveness of the microscope in this particular case.
Scientists have aspired, since the dawn of science, to "see" and "move" individual atoms and particles in order to expand human knowledge and advance the frontiers of research and the preparation of nanoscale components. IBM has been a pioneer in nanoscience and nanotechnology since the development of the scanning tunneling microscope (STM) in 1981 in its laboratories in Zurich. For this invention, which made it possible for the first time to image individual atoms, the two developers received the Nobel Prize in Physics for 1986. The AFM microscope, which is the direct continuation of the STM microscope, was invented by the same scientists in 1986.

One response
Finally, finally 🙂
Very nice