Researchers at the Technion have developed a new method for producing hydrogen from water using solar energy. The new method will make it possible to produce the hydrogen in a concentrated way away from the solar farm in an economical way and efficient
Researchers at the Technion have developed a new approach to producing hydrogen from water using solar energy. In an article published in the journal Nature Materials, the researchers explain that this approach will make it possible to produce the hydrogen centrally at the point of sale - for example, at a gas station for a hydrogen-powered electric vehicle - even if it is far from the solar farm. The new technology is expected to significantly reduce the costs of producing hydrogen and transporting it to the customer.
The research was led by Abigail Landman, a doctoral student in the Grand Technion Energy Program (GTEP), and Dr. Chen Dotan from the Laboratory for Electrochemical Materials and Devices. Abigail is working on her doctorate under the guidance of Prof. Abner Rothschild from the Faculty of Materials Science and Engineering and Prof. Gideon Gerder, Dean of the Faculty of Chemical Engineering. The research was supported by the Center of Excellence (I-CORE) for Solar Fuels Research (funded by the Ministry of National Infrastructures, Energy and Water), the European Joint Venture for Fuel and Hydrogen Cells (FCH-JU), the Energy Program of the Grand Technion ( GTEP), donor Ed Suttle and the Edlis Foundation.
The fuel of the future
Hydrogen is considered one of the most promising energy carriers for vehicle propulsion and various needs, due to its outstanding advantages:
- Hydrogen can be extracted from water, so its production does not depend on access to depleting natural minerals.
- Using hydrogen fuel will reduce the dependence on fossil fuels such as oil and natural gas, the availability of which depends on geographical, political and other factors, and will increase the energy available to the Earth's population.
- Unlike diesel and gasoline engines that emit a lot of pollution into the air, the only byproduct of hydrogen engines is water.
Due to the advantages of hydrogen propulsion, many countries, led by Japan, Germany and the USA, are investing huge sums in programs to develop "green" (environmentally friendly) technologies for hydrogen production. Most hydrogen is currently produced from natural gas in a process that emits carbon dioxide into the air, but it is also possible to produce hydrogen by breaking down water molecules into hydrogen and oxygen in a process called electrolysis ("decomposition using electricity"). However, since the production of electricity itself is an expensive and polluting process, researchers at the Technion and around the world are developing a cell Photoelectrochemical which utilizes the sun's energy to break down the water directly without the need for electricity.
The challenge of separation
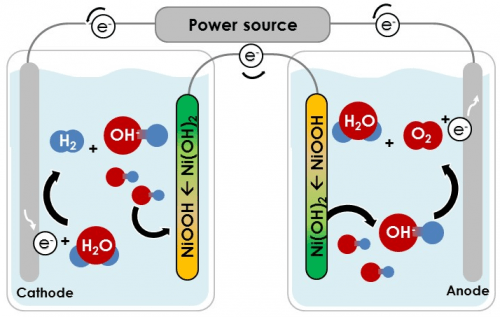
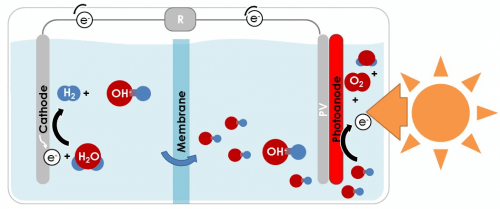
The main challenges in the development of solar farms for the production of hydrogen are the separation between the hydrogen and oxygen, the collection of the hydrogen from millions of photo-electrochemical cells and its transportation to the point of sale. Technion researchers solved the problem by developing a new method for the photoelectrochemical decomposition of water. In this method the hydrogen and oxygen are formed in two separate tanks, which are connected by electrical connections only. This is in contrast to the conventional method, in which the hydrogen and oxygen are accumulated in one container (cell), when a thin membrane separates them and prevents them from mixing with each other. It is worth noting that a mixture of hydrogen and oxygen is flammable and explosive, so this separation is essential.
The new process allows Geographical separation Between the solar farm, which consists of millions of photo-electrochemical cells that produce only oxygen, and the site where the hydrogen is produced in a concentrated, economical and efficient manner. In other words, this method makes it possible to harvest the sun's energy in one place and produce the hydrogen in another - a gas station, for example. All that is required is a pair of auxiliary electrodes from nickel hydroxide, an inexpensive material used in rechargeable batteries, and a metal wire connecting them.
"In the current article we describe the creation of hydrogen using the new method while physically separating the production of hydrogen from the production of oxygen," says Abigail. "According to the cost estimate we conducted, our method can successfully compete with existing methods of water splitting and be a cheap and safe platform for hydrogen production."
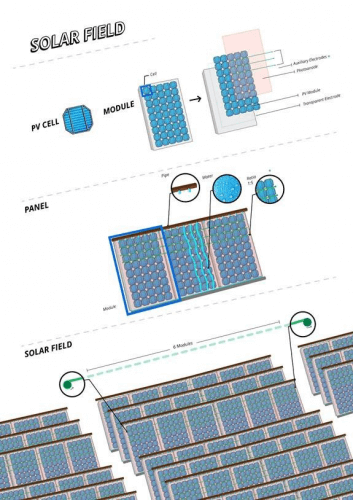
Looking ahead
The method developed at the Technion to separate the production of hydrogen and the production of oxygen formed the basis for the development of a new technology for two-stage electrolysis. This technology, which was developed by Dr. Chen Dotan, enables the production of hydrogen with unprecedented efficiency and high pressure, thus significantly reducing the costs of hydrogen production. Now the new technology is in pre-industrial development stages.
Doctoral student Abigail Landman recently presented the said research in the competition Three Minute Thesis in Australia. This lecture earned her first place in the energy category. In the competition, held at the initiative of the University of Queensland, the participants are required to present groundbreaking research in just three minutes.

4 תגובות
There is no problem to produce hydrogen from water and save up to 60 percent of the fuel... the problem is that water is difficult to collect taxes!!.. so forget about it....
Herzl.. they don't burn hydrogen... read about fuel cells
The whole hydrogen thing is big hype with no real substance behind it.
A. Hydrogen is horrifically explosive. A leak is enough, the explosion is spontaneous and no spark is needed. Hydrogen powered vehicles will not be allowed to enter any city. Gas stations will be death traps.
B. The only way to store hydrogen today is under high pressure. (Did we say explosive?)…. Perhaps in the future they will succeed in developing methods of storing heavy metals inside crystals. I wish
third. Hydrogen damages metals by entering into the metal and making it very brittle. This means exotic and expensive alloys for tanks, piping, etc. Or the lifetime of the piping and tanks will be short.
d. The efficiency of burning hydrogen in an internal combustion engine will be 35-40%. Fuel cells will get closer to 100% but they are very expensive.
So what's left? Production of hydrogen and its storage in desert areas far from people, for energy storage during the day and use at night. Maybe in the future after they solve the problems we mentioned above.
So what will the Saudis do with the oil????????