Scientists have discovered for the first time ever an ionic crystal consisting of only one chemical element - boron. This is the most concentrated and strongest form of this element that usually hides in compounds, which has not been successful despite the attempts of the best chemists for over 200 years
Scientists have discovered for the first time ever an ionic crystal consisting of only one chemical element - boron. This is the most concentrated and strongest form of this element. It turned out that the new shape is a key to understanding Bohr's phase diagram - the only element whose phase diagram has been unknown since its discovery two centuries ago.
This research was published in the prestigious scientific journal Nature. Findings on the strength of the new shape are published in a separate article in the scientific journal Superhard Materials.
The team of authors was composed of sub-teams headed by researchers Artem R. Oganov (theoretical crystallographer from Stony Brook University), Jiuhua Chen (materials researcher from the University of Florida), Carlo Gatti (theoretical chemist from the University of Milan in Italy) and Vladimir Solozhenko (theoretical chemist from the French research institute CNRS ). Such a massive effort is required to crack what is probably the most complex element in the periodic table.
Bor has long been known as the scientific "graveyard" of great researchers. Its strange story began back in 1808 when two excellent teams, one from Paris and the other from London, separately announced the discovery of a new element, boron. It was later proven that in both cases the "element" was actually a compound that contained no more than seventy percent boron. The most comprehensive proof of this was obtained by another excellent chemist, H. Moissan, but his material was also later proven to contain no more than ninety percent boron.
In 1858, the scientist F. Wöhler wrote in his book that boron has two forms, polymorphs - one graphite-like and the other diamond-like. Today we know that both forms are actually compounds, AlB12 and B48C2Al, respectively. The first time a pit was prepared at the ninety-nine percent level was in 1909, but that was not the end of the story. Even one percent of impurities, or less, can change the general structure and properties of boron very sharply and even compounds such as PuB100 are known.
"Such susceptibility to contamination is unprecedented among other elements and makes the study of this element nothing less than a nightmare," says Artem R. Oganov, associate professor in the Department of Geosciences at Stony Brook University.
To date, about sixteen polymorphic forms of boron have been reported, but it is likely that most of them are contaminated but stable forms. It is the only element whose ground state is not known experimentally. Among Bohr's other anomalies, it has recently been suggested that it violates the third law of thermodynamics (which states that stable forms at absolute zero must be perfectly ordered) at atmospheric pressure. The pit's behavior at high pressures remains even more mysterious.
The infrastructure for the current study was prepared in 2004, when researchers Chen and Solozhenko independently synthesized a new form of pit at high temperatures and pressures above one hundred thousand atmospheres. It was not possible to determine the exact structure of the new shape based on the experimental findings alone and a new theoretical method developed by the researcher Dr. Oganov at the time was required.
"The method is purely theoretical and does not require any experimental information, and is based on principles of natural development applied to the most stable crystal structure," says the researcher. "The computer produces dozens of theoretical crystal structures, whose energy is estimated through calculations of quantum mechanics, and then the most stable structures are "paired" with each other and "give birth" to additional structures until the most stable structure is obtained."
Using this method, Dr. Oganov was able to find the correct structure and determine that it is indeed the ground state of boron and that there is a significant charge transfer between the different boron atoms in the structure.
Advanced analysis by Dr. Gatti confirmed the existence of an ionic state between the bonds and further experiments by Dr. Solozhenko determined that the new form is extremely strong and has a Vickers hardness value of 50 GPa, compared to diamond, the strongest natural material, valuable of 70-150 GPa. Calculations in quantum mechanics suggest the existence of an extensive region of stability for this new form, extending even to pressures of nine hundred thousand atmospheres.
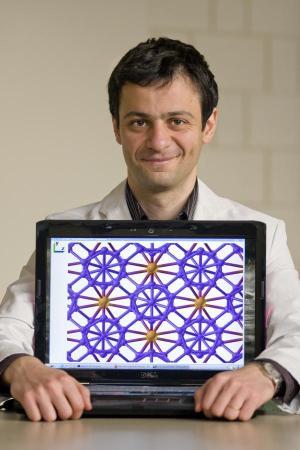
How is it possible for a foundation to be June? Basic chemistry books teach that charge transfer occurs when atoms have different electronegativities and this fact automatically rules out the possibility of the existence of elements in the ionic state. Burr found a surprising and fascinating solution to this truth - his new structure consists of two very different types of nanoclusters - an icosahedron (a body with twenty faces) B12 (seen in blue in the drawing above) and a B2 cluster (appearing in orange in the drawing above). The electronic structure of these two clusters is very different - in fact, the dependence of the electronic properties on the size of the cluster is known and serves as an important principle in nanotechnology. The electronegativity of the two types of clusters is different and this fact gives rise to charge dispersion and the acceptance of partial ionic character in this interesting structure.
"Another amazing fact," explains Dr. Oganov, "lies in the fact that the centers of mass of the clusters in this new structure occupy the same positions of the atoms in the structure of table salt (NaCl) - the archetypal ionic structure.
As a result of these findings, the researchers predict the existence of other ionic forms for additional elements and suggest several stable or semi-stable possibilities. In addition to this, it is likely that in elements that are liquids there is a certain level of momentary charge dispersion between the atoms. Besides being intriguing, ionic elements have interesting and important properties. The properties most affected by ionic character include dielectric constants, vibrational spectrum and the gap ratio between the electron bands. Among the unusual properties of this new structure, the predicted infrared absorption spectrum (due entirely to a different distribution of the electronic charge) has already been fully confirmed by Chen's team's experiments.
The news about the study on behalf of a university Stony Brook

2 תגובות
It is interesting how common this phenomenon is of conformational inventions that allow this ionic state. I wonder if this opens the door to stronger materials than diamond.
WOW…
interesting