"In the future, we will be able to create a device that contains one type of functionality in one area and completely different functionality in another area, in the same material. These areas will work differently, but they will still be part of the same compact and cheap device," explains the lead researcher.
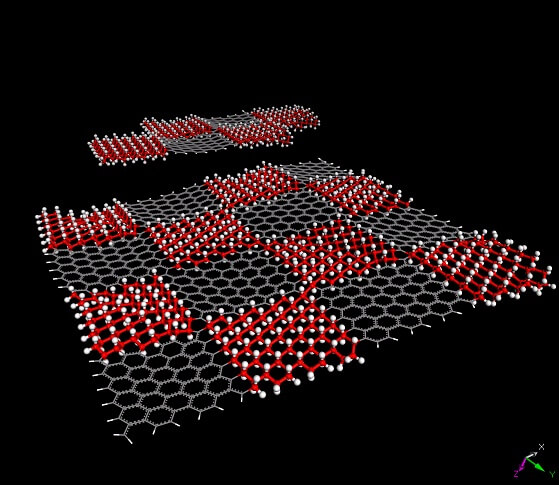
The future for organic chemistry has become more expansive after Rice University researchers discovered a highly controlled way of attaching organic molecules to pure graphene, a method that allows the material to be suitable for a variety of new applications.
A team of researchers in the laboratory of chemist James Tour at Rice University, relying on previous findings in graphene research, developed a two-step method to transform the innovative material graphene (a single-atom sheet) into a superlattice for its use in organic chemistry. The research findings could lead to progress in the development of graphene-based chemical sensors, thermo-electronic devices and completely innovative materials. The research findings were published in the scientific journal Nature Communications.
Graphene itself is inert in many organic reactions, and as a semi-metal, it lacks any band gap; This fact limits its usefulness in the field of electronic components. However, the new research demonstrated how graphene, the hardest known material other than diamond, could be suitable for new types of chemistry.
Until now, there was no way to attach different molecules to the plane of the graphene sheet, says the lead researcher. "In most cases, they would reach the edges, and not the center of the material," he adds and says. "However, using our new two-step method, we are able to cause graphene to undergo a Memnon reaction (hydrogenation, introduction of hydrogen atoms) in order to build a defined template and in the next step connect other molecules to the positions where the hydrogen atoms were present.
"Our method is useful for the preparation of, for example, chemical sensors where you want proteins, DNA molecules or sugars to be attached outside the graphene sheet at defined positions across the device. The reactivity in these positions is particularly fast compared to the connection of molecules to the edges of the graphene. Now we have the possibility to determine what their connection point will be."
The first step in the process involves the preparation of a lithographic template that allows the connection of hydrogen atoms to defined positions in the graphene sheet; The researchers turned the one-dimensional surface into a two-dimensional superlattice, a semiconductor called graphane. The hydrogen atoms were inserted using a heated filament using an approach developed by fellow researcher at Rice University, Robert Hauge.
The researchers demonstrated their ability to inject into graphene precisely processed graphene islands while engraving microscopic text and the Rice University logo. Graphene normally repels fluorescent molecules, but graphene doesn't work that way, so the symbol actually glowed when we irradiated the graphene sheet. This new method allows researchers to see patterns at a high resolution of nearly one micron, the limit of conventional lithography available to science today.
In the next step, the researchers exposed the material to diazonium salts which chemically "attacked" the carbon-hydrogen bonds of the formed islands. These salts had the fascinating possibility of "eliminating" the hydrogen atoms while obtaining carbon-carbon bonds that allow them to combine with other organic substances. "What we got in this research is the ability to move from the graphene-graphene superlattice to a hybrid material that is a much more complex superlattice," explains one of the researchers. "We are interested in causing functional changes through which it is possible to control the location and types of chemical bonds, the functional groups and the concentrations of the various substances.
"In the future, we will be able to create a device that contains one type of functionality in one area and completely different functionality in another area, in the same material. These areas will work differently, but they will still be part of the same compact and cheap device," explains the lead researcher. "At the beginning of the graphene era, it was not possible to apply a lot of organic chemistry on it. Now, we can do almost anything with it, in terms of organic chemistry. Our method opens a window to many possibilities and applications."

2 תגובות
to AR
It is not always easy to find good problems for solutions. There are examples
For great solutions that have waited many years for suitable problems.
A laser for example, quasi-crystals for example and possibly even graphene.
Application examples please