A new observation could lead to improved industrial production of high-quality graphene, ushering in the era of graphene-based electronics, thanks to engineers at the University of Illinois
A new observation could lead to improved industrial production of high-quality graphene, ushering in the era of graphene-based electronics, thanks to engineers at the University of Illinois.
Using a combination of information derived from several imaging methods, the research team found that the quality of the graphene depends on the crystalline structure of the copper substrate on which it is produced. The group, led by computer and electronics engineering professors Joseph Lyding and Eric Pop, published their research findings in the scientific journal Nano Letters.
"Graphene is a particularly important material," says Lyding. "The future of the electronics field may depend on it. The level of quality of its production is one of the still unsolved problems in the nanotechnology sector and the findings of our research constitute one more step in the trend toward a solution."
In order to produce extensive sheets of the material graphene, methane gas is injected into a furnace containing a sheet of copper foil. When the methane hits the copper, the carbon-hydrogen bonds in it are broken, the hydrogen is released in the form of a gas while the carbon atoms bind to the copper surface, where they move until the moment they connect to each other and thereby form the graphene layer. Copper is a sought-after substrate because it is relatively cheap and because it is able to promote the creation of single-layer graphene, a product that is important for electronics applications. "This is an economical and direct way to prepare graphene on a large scale," notes one of the researchers. "However, this process does not take into account the intricacies of the graphene creation mechanism," he adds. "Understanding these subtleties is important for the production of high quality electronic components with a high level of performance."
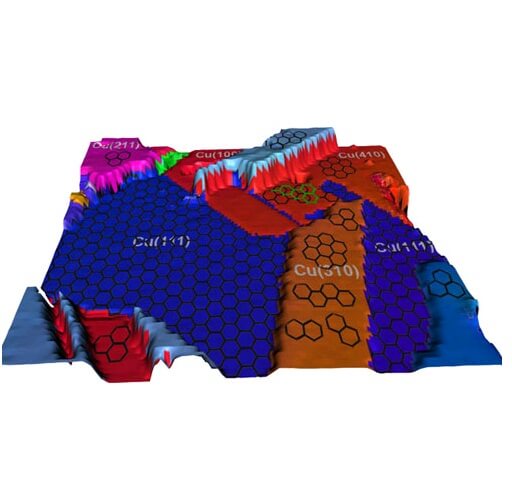
Although graphene produced on copper tends to be of higher quality than graphene produced on other types of substrates, it is still riddled with defects and multilayer regions, two facts that prevent it from being used in high-performance applications. Researchers thought that the natural roughness of the copper surface might affect the acceptance of the graphene, but the research group from the University of Illinois found that the crystalline structure of the copper is the more important factor. Copper foils are patchwork and have different crystal structures. From the moment the methane reaches the surface of the foil, the shapes of the copper crystals it meets affect the quality of the graphene that will be obtained from the carbon atoms.
Different crystalline forms are assigned specific numerical values. Using several advanced imaging methods, the research team found that copper substrates with higher index numbers tend to produce lower quality graphene. In addition, he discovered that two common crystal structures, labeled (100) and (111) lead to the worst and best quality, respectively. The crystals with the (100) structure have a cube shape and have large spaces between the atoms, while the (111) type crystals have a densely packed hexagonal structure.
"In a structure of the (100) type, there is a higher probability that the carbon atoms will sink into the holes found in the copper at the atomic level, and in the next step they will connect vertically with each other, than they will fly out and connect with each other in the transverse axis," explains the researcher. "The (111) surface is hexagonal, and so is the graphene itself. This does not mean that the match is perfect, but that there is a preferred match between these two surfaces."
Researchers are now faced with the issue of balancing the price of only (111) type copper against the value of high-quality defect-free graphene. Although it is possible to produce monocrystalline copper, the process is complex and expensive.
The researchers estimate that it is possible to improve the production processes of the copper foils so that they lead to obtaining a higher amount of crystals of the desired type - (111). Graphene produced on such foils will not be ideal, but it may be "good enough" for most applications.
"The question is how do you optimize the processes while maintaining financial efficiency for technological applications?" asks the researcher. "As a community, we are still writing the "cookbook" for making graphene. We are constantly refining our methods and trying new recipes. As with any technology in its infancy, we are still testing what works and what doesn't."
In the next step, the researchers hope to use their method to test the preparation of other two-dimensional materials besides graphene, including insulating materials, in order to improve the performance of graphene-based devices. In addition, the researchers plan to make graphene on single-crystal copper.
"There is a lot of uncertainty in the graphene business today," notes the lead researcher. "The fact that there is a clear difference in the observations between these two different preparation mechanisms helps to direct the research and may allow conducting more quantitative experiments and developing better models. Our article steers things in this direction."

2 תגובות
It's been a long time since graphene was written here, I started to miss it.