The new research improves our understanding of the chemistry required to create hydrogen fuel from water
Single oxygen atoms "dancing" over a metal oxide surface have helped chemists understand how water separates into hydrogen and oxygen atoms. In the process, the scientists were able to describe a chemical reaction that was previously only theoretical. The new research improves our understanding of the chemistry required to create hydrogen fuel from water.
The scientists came across the discovery while trying to determine the basic rules by which titanium dioxide - a compound sometimes found in suntan cream - breaks apart water into its atomic components. The chemical reactions between water and oxygen are central to diverse processes such as: hydrogen production, pollutant decomposition and solar energy.
"Oxygen and water are involved in many processes," said physicist Igor Lyubinetsky of the Department of Energy at the Pacific Northwest National Laboratory, who reported the research team's findings in the scientific journal Physical Review Letters. "This mobility may hinder some responses and help others."
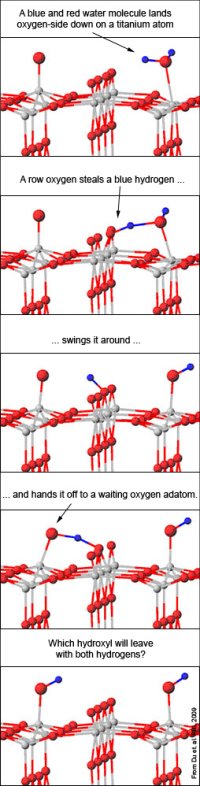
During the examination of the fission of water into oxygen and hydrogen using titanium dioxide, the researchers can use a method called "Scanning Tunneling Microscope, STM, a microscope with which surfaces can be examined at the atomic level) to observe the chemical reaction. The surface of titanium dioxide resembles a cornfield: a row of oxygen atoms sticking out from a row of titanium atoms. The rows of oxygen and titanium that appear in the stitches are similar to a kind of strips.
The scientists are also able to see certain atoms that "stop" on the surface as bright spots. Such a visible atom is a single oxygen atom located on a titanium atom, and is called an "adatom" (an atom located on a crystal surface, short for the term "adsorbed atom"). Chemists are only able to distinguish water droplets if their temperature is extremely low.
In the current study, the scientists examined the reactions of water with titanium dioxide at normal temperatures. They started with a surface coated with a few adsorbed oxygen atoms, then added water, and then the oxygen atoms began to "dance".
"Suddenly, almost all of these atoms began to move back and forth along the titanium row," the researcher said. Surprisingly, these atoms not only glided up and down across the strips but also bounced out of the titanium atoms and landed next to them, as if in a wild dance."
"We saw completely unexpected things. We thought it was very strange - we saw adsorbed atoms that simply jumped from one titanium atom to another," explains the researcher. "We just couldn't explain it."
In calculating the amount of energy these atoms would need to move on their own, and jump from one titanium atom to another, the chemists suspected that these atoms had additional assistance - probably from the unobserved water particles.
To explain the phenomenon, the team calculated the energy needed to propel these loose atoms with the help of the water molecules. If a water molecule is positioned near an adsorbed oxygen atom, one of the water's hydrogen atoms is able to jump to it and thereby create two oxygen-hydrogen pairs. These pairs are known as hydroxyl groups and tend to "steal" atoms from other atoms, including each other. One of these groups is able to steal a hydrogen atom from a neighboring identical group and become a water molecule again. This water separates away from her, leaving behind an adsorbed atom.
The chemists determined that water is able to help such an atom to jump between rows as well: if a water atom and an adsorbed atom are located on either side of an oxygen row, such oxygen can act as an intermediary in transferring one hydrogen atom from the water atom to the other adsorbed atom. Again, two hydroxyl groups are formed, one of which, in the end, "steals" the two hydrogen atoms (with the help of the mediator) and moves away from the surface.
The energies calculated for these two different scenarios best fit the researchers' experimental data. When a row of oxygens is used as an intermediary, the process is called "pseudo-dissociation", a reaction proposed by chemists but never experimentally confirmed - until now.
"We realized that only if we include in our calculations this situation of simulated separation of water we can explain the entire phenomenon," explains the researcher. "Otherwise, all the calculations show that there is an energy barrier that is too high - the adsorbed atom simply cannot detach on its own."
The researchers emphasize that in this mechanism the water is used as a catalyst. A catalyst is a substance that aids a chemical reaction without changing itself. "The water is needed to move the adsorbed atoms from place to place, but like a catalyst, it is not consumed during the reaction," he explains. "You start with water and end with water."
In the next step, the researchers plan to test whether water is able to move adsorbed atoms of other types. In addition, they will examine how radiation affects this mechanism.
