After observing nature and receiving inspiration from it, researchers used a common protein to guide the design of a material capable of storing hydrogen gas inside.
After observing nature and receiving inspiration from it, researchers used a common protein to guide the design of a material capable of storing hydrogen gas inside. The synthetic material works ten times faster than the original protein found in bacteria.
This step is only one part of a series of reactions aimed at splitting the water molecule to form hydrogen gas, but the researchers note that the findings demonstrate that they are able to learn from nature how to control these reactions in order to develop stable synthetic catalysts for energy storage, for example for the development of fuel cells. In addition, the natural protein, which is an enzyme, is composed of cheap and common metals, a characteristic adopted by the researchers. Today, these materials, called catalysts, are based on precious metals such as platinum.
"This catalyst, based on the metal nickel, is really fast," notes principal investigator Morris Bullock of the Department of Energy's National Laboratory of the US Department of Energy. "It is about a hundred times faster than the fastest catalyst known so far. And we know from nature that it will be possible to produce it using common and cheap metals such as nickel or iron."
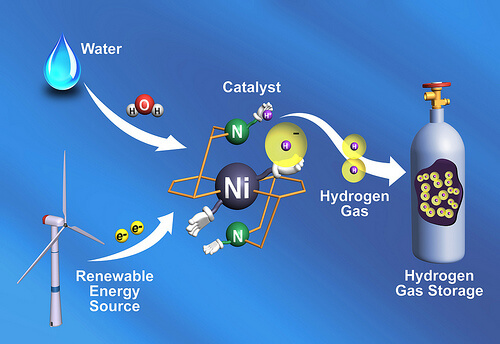
Electrical energy is nothing but the movement of electrons. Those electrons are also responsible for binding the atoms together when they are chemically bonded to each other in molecules such as hydrogen gas. Converting electrons into chemical bonds is one of the ways to store electrical energy, a method that is especially important for renewable and sustainable energy sources, such as solar energy or wind energy. The conversion of the chemical bonds back into flowing electricity, when the sun is not shining or when the wind is not blowing, allows the utilization of the stored energy, such as in a fuel cell powered by hydrogen. Normally electrons are stored in batteries, but the researchers are interested in taking advantage of the tighter packing available in the materials themselves.
"We are interested in storing energy in the most compressed way possible. Within chemical bonds, a huge amount of energy can be stored in a small physical volume," explains one of the researchers. Biological systems store energy in a compressed form all the time. Plants use photosynthesis to store the sun's energy in chemical bonds, which humans use when they consume food; And common bacteria store energy in the chemical bonds of hydrogen gas with the help of a protein known as hydrogenase. Since this natural enzyme does not extend life as much as man-made synthetic enzymes (eg, paper vs. plastic), the researchers wanted to take out the active part of the biological protein and redesign it with a more stable chemical skeleton.
In this study, the scientists focused on only a small part of the process of splitting water into hydrogen gas, like fast-forwarding to the end of a movie. Among the many steps, there is a step where the catalyst is bound to two hydrogen atoms taken from the water.
The catalyst does this by completely removing certain hydrogen atoms from a hydrogen source such as water and attaching them to each other. Given the simplicity of hydrogen atoms, these components are positively charged protons and negatively charged electrons. The catalyst arranges these components in the right direction so that they can react with each other in the most appropriate way. "Two protons and two electrons are equal to one molecule of hydrogen gas," explains the researcher. In real life, the protons would come from the water molecule, but since the team only looked at part of the reaction, the researchers used water substitutes such as acids to test its catalyst.
In accordance with the mechanism of the natural protein's activity, the experimental catalyst includes regions that protrude outside the central structure and whose role is to attract protons, and are called "pending aminos". This pendant amine moves the proton to a position at the tip of the catalyst, while the nickel atom at the center of the catalyst provides a hydrogen atom with an excess electron. The hydrogen held by the amine group is positively charged, while the nickel atom holds a negatively charged hydrogen atom. Being placed close to each other, the two oppositely charged particles are attracted to each other and coalesce into a molecule of hydrogen gas.
With this plan in mind, the team built possible catalysts and tested them. In their first attempt, they attached a collection of dangling amines around the nickel core, thinking that the more, the better. When they tested this catalyst, they found that it did not act very quickly. Analysis of the mechanism by which the catalyst moved the protons and electrons suggested that too many dangling amine groups interfered with the complete reaction. Too high a concentration of protons slows down the reaction that produces the hydrogen gas. Following this, the team of researchers removed a number of reliable groups from the catalyst and left the minimum number necessary for its activity.
When the researchers tested the new catalyst, they found that it worked much better than expected. Initially they tested the catalyst under conditions where there was no water at all and the catalyst produced hydrogen gas at a rate of 33000 molecules per second. This rate is much faster than the natural enzyme which is 10000 molecules per second.
However, in most everyday applications water will be present, so they added water to the reaction to test how it worked. The catalyst worked three times faster and produced 100000 hydrogen molecules every second. The researchers believe that the water molecule may help by moving the protons to advantageous positions in the amine group, but they are still examining the details of the mechanism. At the same time, the new catalyst has a drawback - it is fast, but it is not efficient. Researchers have several ideas for how to increase efficiency. In addition, future research will require the preparation of a catalyst that will be able to split a water molecule in addition to creating hydrogen gas.

2 תגובות
Amazing. It's simply amazing how people don't understand the magnitude of the challenge and the huge limitations in switching from fossil fuels to renewable sources. They do not understand how oil is a unique and extremely difficult to replace energy source.
Still, it's a bit annoying that there are almost no articles about research in renewable energy. Just a bit of hydrogen here and there. Is it because there are almost no breakthroughs in the last year?
Amazing. It's simply amazing that humanity is still stuck on polluting fossil fuels and not switching to sustainable energy sources.
What is required of us to understand that our existence and the quality of our lives (and that of other companies) depend on this transition.