Catalysts convert unwanted or useless chemicals into more useful or desirable substances. A study, published this month in the scientific journal Science, reveals important details of a common catalyst
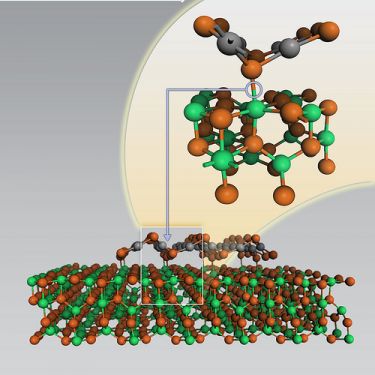
Catalysts convert unwanted or useless chemicals into more useful or desirable substances. A study, published this month in the scientific journal Science, reveals important details of a common catalyst: how a raft-like structure of chemically active platinum is formed in the catalyst. The new research has yielded new insights to improve the effectiveness of a common industrial catalyst used in oil refining, chemical processing and environmental uses.
The research shows that aluminum atoms in the supporting substrate, which require an additional bond, capture and anchor themselves to the platinum atoms. The anchors allow the platinum atoms to assemble into rafts that "float" above the supporting surface while providing plenty of space for the catalytic reactions.
Researchers from the US Department of Energy performed the test for an industrial catalyst known as platinum anchored on aluminum oxide. These expensive combinations of metal and oxide are the most common type of industrial catalysts. The new research will help engineers control the preparation of this catalyst, a control that will lead to improved performance. "We were able to precisely locate an important site for the anchoring of platinum to the surface of the aluminum oxide obtained during the preparation," said chemist Chuck Peden, one of the co-authors of the paper. "Despite the fact that platinum raft structures have already been observed in the past, this is the first time we have received a picture on a molecular scale of the processes that create them."
In these catalysts, the oxides are nothing more than a surface on which the precious metals are placed while they cleave and form bonds in other compounds, such as those used in car exhausts. The most effective catalysts spread the atoms of the precious metals uniformly over the surface of the oxide. In the ineffective catalysts the metal atoms become entangled clumps, so that the inner atoms are no longer available to do their work with the other atoms.
Chemists working with this catalyst have previously reported that under certain conditions rafts of platinum atoms may form on the oxide surface. Unlike atomic blocks, rafts expose most of their atoms to other particles, a fact that makes them highly desirable structures. However, in order to control the production of the raft structures, the researchers had to learn how they are formed.
To this end, the researchers prepared aluminum oxide surfaces under typical synthesis conditions and tested them before and after coating with platinum atoms. Initially, they examined the chemical nature of the surface with the help of one of the most powerful devices of its kind in the world - a 900 MHz NMR spectrometer. The device provided an unprecedented level of resolution of the aluminum oxide structure, allowing researchers to identify aluminum atoms with unique properties. Due to their chemical nature, aluminum atoms tend to bond to four or six atoms. The researchers discovered, on the other hand, that in the absence of platinum atoms, some of the aluminum atoms were bound to five atoms, which constitute a less "convenient" structure. However, adding platinum atoms to the mixture resulted in a reduction in the number of aluminum atoms bonded to five other atoms, and an increase in the number of those bonded to six atoms. The number of aluminum atoms bonded to four other atoms remains constant, a fact that suggests that the platinum atoms are located at closer points than the atoms bonded to five atoms, called penta sites. The research team discovered that the number of penta sites can be increased by increasing the temperature during the preparation of the catalyst. More penta sites means more platinum atoms anchored to the surface.
At the end of their series of experiments, the researchers showed that penta aluminum atoms are essential for the formation of the raft structures. Aluminum oxides do not originally contain penta ethers. When the researchers examined catalysts prepared from aluminum oxide under a microscope, they saw that the platinum atoms formed spherical clumps that oscillated on the surface instead of organized raft structures.
Theoretical calculations that took into account all the experimental information produced a computer model of the catalyst's formation mechanism. These findings provided insights into the most appropriate way to prepare more effective catalysts.
"The understanding we achieved in the research gave rise to a number of additional tools that can be used to obtain a better and more uniform distribution of the platinum atoms," the researcher notes. "For example, if we can find conditions where platinum atoms can be added at higher temperatures than exist today, then we can have many more binding sites available." Finding the exact conditions that will allow chemists to control the number and degree of dispersion of the pentasites will be the subject of future studies by this group.
