Physical chemists from China have created carbon-50 molecules in the solid state for the first time. Carbon-50 can easily react with a variety of organic groups to form new compounds with interesting chemical and physical properties.
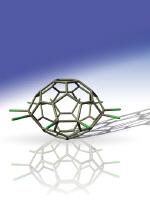
Physical chemists from China have created carbon-50 molecules in the solid state for the first time. Lan-Sun Zheng, his colleagues at Zeeman University and his colleagues at the Chinese Academy of Sciences in Beijing Wan prepared the molecules, which are considered the lost little sister of the carbon-60 molecule, using a chlorine-assisted charge-discharge technique. The results of the research will allow scientists to study the properties of the carbon-50 molecule and take advantage of its special properties. The method, developed by the Chinese team, paves the way for the creation of other small cage-shaped carbon molecules (fullerene molecules).
The most popular cage molecule is carbon-60, which is also known by the names "buckminsterfullerene" (buckminsterfullerene after the architect Richard Buckminster Fuller, D.A) or "buckyball". The molecule, which consists of sixty carbon atoms arranged in pentagons and hexagons, was first created in 1985. Since then, larger cage molecules have been created, containing 70 and even 500 carbon atoms.
All these molecules, created so far, obeyed the "isolated pentagon rule" (IPR - Isolated Pentagon Rule), which states that the most stable molecules are those in which each pentagon is surrounded by five hexagons. However, this condition cannot be fulfilled in molecules with less than sixty carbon atoms. This means that caged molecules that do not obey the IPR rule will have unusual properties. Also, it makes their structure unstable, and difficult to synthesize. Until now, cage molecules with less than sixty carbon atoms have only been produced in the gaseous state.
Zeng and his colleagues were able to stabilize carbon-50 molecules in a solid state using a graphite arc-discharge method. They added carbon tetrachloride vapor at a pressure of 0.013 atmospheres to helium at a pressure of 0.395 atmospheres in a sealed stainless steel vessel. Then apply an electric field of twenty-four volts. Next they cleaned 90 grams of soot, which contained carbon-50 chloride (C50Cl10) and were left with two milligrams of 50% pure carbon-99.5 chloride.
"The carbon-50 caloric molecule looks like a spaceship or a spinning planet. It has 10 carbon chloride arms ready and waiting for chemical action,” team member Su-Yuan Xie told Physics Web (see figure). Like carbon-60 and carbon-70 derivatives, Zee says carbon-50 can easily react with a variety of organic groups to form new compounds with interesting chemical and physical properties. Beyond that, it will be possible to use the method to synthesize other small cage molecules, such as carbon 54 and carbon 56.
Translation: Dikla Oren
