Weizmann Institute scientists and their research partners have shown that anti-freezing proteins can also promote freezing
Living things can survive particularly harsh winters with the help of antifreeze materials. These proteins help, among other things, fish, insects, plants and even bacteria to withstand extremely low temperatures; If it weren't for those proteins, their liquid contents would freeze, and they would die. Amazingly, under certain cold conditions, the antifreeze proteins can promote freezing. These findings were recently discovered in experiments conducted in Israel and Germany with proteins derived from fish and beetles. The results of the research, published in the scientific journal Journal of Physical Chemistry Letters, may have implications for understanding the basic processes of ice formation.
Antifreeze proteins do not prevent ice from forming in the first place. In fact, they bind to the tiny ice "nuclei" that form in the living cell, preventing them from turning into larger crystals. These proteins are found, for example, in the exoskeleton of flour beetle larvae and protect the larvae's vulnerable skin. In nature, there are also proteins that do exactly the opposite: promote ice formation. Certain bacteria, for example, can use these proteins to form sharp ice crystals that injure the skins of fruits or leaves. Despite the plausible explanation that these are completely different types of proteins, previous studies indicated a certain similarity between them. The basic premise of the studies was based on the fact that antifreeze proteins have an active site that can bind to ice - and therefore their ability to assist in the formation of an initial ice nucleus that may later grow into an ice crystal. However, so far the way to fully characterize the activity of these biological molecules has not been found.
Antifreeze proteins are currently used in the food industry - for example, to maintain the smooth texture of ice cream and keep ice from its outer surface
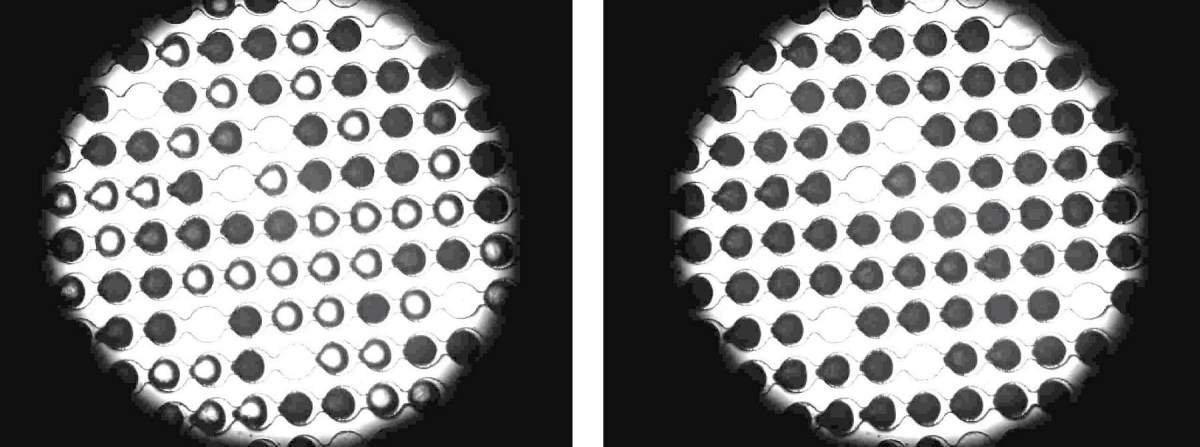
The current research was led by Prof. Thomas Kopp from Bielefeld University in Germany in collaboration with Prof. Ido Breslavsky from the Hebrew University of Jerusalem and Prof. Yanon Rodich from the Weizmann Institute of Science. The research was made possible thanks to a microfluidic device developed by Dr. Naama Reicher from Prof. Rodich's research group that allows capturing extremely tiny drops of very clean water using a microarray of chips. To these microdroplets, the researchers added well-measured amounts of antifreeze proteins isolated from flour beetle larvae or fish living in the Arctic region. This experimental setup allowed the research group to verify that the ice formation is induced by the proteins.

During the experiment, the captured microdroplets remained liquid even when the temperature dropped below zero, as they did not have the same particles and contaminants that cause water to freeze at 0 degrees Celsius. It was only around 38.5 degrees below zero that the water drops that were not added to them began to freeze, while in about half of the samples to which antifreeze proteins were added, ice crystals began to form at a higher temperature - close to minus 34 degrees. In other words, the experiment showed that at certain temperatures, which are indeed extreme but not unknown on Earth, antifreezes actually become antifreeze promoters. Naturally, proteins that promote ice formation are able to form ice efficiently at higher temperatures. The scientists hypothesize that the main difference between the types of proteins lies in their size: pro-freezing proteins are significantly larger.
Antifreeze proteins derived from fish are currently used in the food industry - for example, to maintain the smooth texture of ice cream and keep ice from its outer surface. The current research points to the possibility that these proteins may have limitations and, in fact, may promote ice accumulation when exposed to very low temperatures, such as those experienced by the North American continent this year. Proteins that promote the growth of ice crystals have their own uses—for example, at ski resorts that want to extend their ski season, so this research on antifreeze proteins may also point to ways to create improved proteins that promote freezing. For Prof. Rodich, whose research focuses on the atmosphere and climate, the findings may shed light on the physical processes that influence the creation of clouds.

One response
interesting.