The Ogmedics company, which was founded a year ago and won first place in the "BizTech" competition at the Technion, raised NIS 3 million from the TerraLab incubator in Kneam * ReWalk launches sixth generation exoskeleton
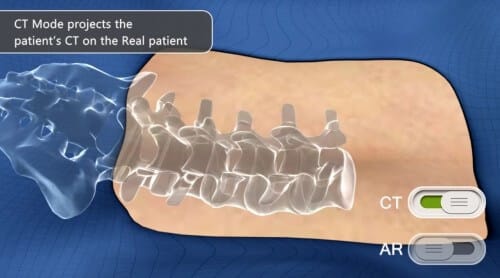
The TerraLab incubator, operating as part of the Chief Scientist's incubator program, invests NIS 3 million in Augmedics LTD - a company established as part of the BizTech competition at the Technion, and won first place in BizTech 2014.
The system that Augmedics is developing - ARGUS - helps surgeons improve the level of surgery and its safety. During the operation, the surgeon wears augmented reality glasses, which project simulated XNUMXD objects onto the patient. For example, in orthopedic surgery, the system presents the surgeon, in real time, with an image of the patient's bones in their actual position and allows him to perform the surgery precisely, as if he saw the bones through the skin. The system is demonstrated in the following video:
With Ogmedix winning the competition last year, the company's CEO, Nissan Elimelech, said that "the competition equipped us with practical tools for formulating the business plan and with practical help from the mentors, and first and foremost gave us a framework and got us into a work routine. All this helped us in developing the technology and planning the future directions in the development of our product, ARGUS."
Last week, the finalists were chosen for the BizTech 2015 finals. Out of 85 teams that submitted nominations for the competition, 12 were chosen, including Renty - a paid equipment lending platform that connects lenders and seekers; Genees - versatile Arduino-based robots that can be controlled from the smartphone; LabSuit - an innovative system for managing inventory and selling materials for laboratories in the field of chemistry and biology. and Infuse - a software-based solution (only) for navigation inside buildings.
ReWalk launches the sixth generation of the exoskeleton - for use at home and in the community
The Israeli company ReWalk Robotics, a manufacturer of motorized exoskeleton systems, is launching the newest version of the personal system, the ReWalk Personal 6.0. The sixth generation of the system offers spinal cord victims an upgraded product, which provides, among other advantages, the highest walking speed and the most precise adjustment to the user. This is the newest version of the ReWalk system, which received FDA approval, and is intended for use both at home and in the community.
"The launch of version 6.0 gives spinal cord injuries an exoskeleton, designed in a way that better fits the user, will allow faster walking and improved step control compared to the previous generation," said ReWalk CEO Larry Jasinski. "Our planning efforts are based on many years of use by the users of the system and a philosophy that advocates improving the product, so that it fits all aspects of everyday life. The design provides customization at the highest level through software that allows for adjustment to the measurements and anatomy of each user individually. All of these improve the user experience compared to the previous versions and compared to other exoskeleton systems, which are in clinical trials around the world."
The advantages of the ReWalk 6.0 system include: a very precise adjustment - to the user's measurements and production according to personal specifications, which allows for improved function, safety and balance between the user's joints. Higher walking speed - Clinical studies conducted with the ReWalk device have shown that users of the system are able to walk at a speed of 0.71 meters per second, faster than any other exoskeleton system. More efficient design - removing the back assembly reduces the weight placed on the shoulders and gives the user freedom in choosing clothes and movement. The new and clean design of the straps and padded surfaces allows the user to wear and remove the system more easily and with improved speed.
"The experience we've gained in daily use of ReWalk systems and the feedback we receive from the community, combined with technical advances by our research and development team have made it possible to develop and manufacture the next generation of prosthetic exoskeleton," said Judy Geriki, director of marketing and training for ReWalk. "User feedback on the 6.0 version shows that they enjoy an improved fit, better stride control and more comfort, thanks to the removal of the back assembly in the improved model."
ReWalk is the only manufacturer of exoskeletons in the USA that holds FDA approval for both the personal system (Personal) and the rehabilitation system (Rehabilitation) of the product. The company also received regulatory approval for the systems it manufactures in other regions of the world, including North America, Europe, Asia, the Middle East and Australia. The ReWalk is the most widely used exoskeleton around the world, with systems for personal use and systems for use in rehabilitation centers in several countries.
The HBL Hadassit Bio group reports on patent approvals for the Selcure subsidiary
The Israeli company Selcure Neurosciences from the stock market HBL group, which is owned by Hadassit Bio by 21.2%, received approval to register a patent in the USA entitled: METHODS OF CULTURING EMBRYONIC STEM CELLS AND CONTROLLED DIFFERENTIATION. The patent is owned by ES Cell International, which granted Selcure an exclusive license to use the patent. The patent refers to methods for directing the differentiation of embryonic stem cells and their maturation into progenitor cells and is important in protecting Selcure's technology to create mature cells such as nerve cells for transplantation and regeneration.
Selkiur's OpRegen product is intended for the treatment of the retinal degeneration disease Dry AMD, containing pigment cells (RPE) of the retina extracted from embryonic stem cells of human origin using a patented cell differentiation method. Selcure received FDA and Ministry of Health approvals to begin clinical trials of the OpRegen product as well as approval from the Helsinki Committee at the Clinical Research Center in the Hadassah Medical Center in Jerusalem to conduct clinical trials 1 and 2
Kamada announces publication in the scientific journal Pediatric Diabetes
Kamada, a pharmaceutical company that produces products based on plasma proteins and focuses on orphan drugs, reports the publication of positive data in the professional journal Pediatric Diabetes from the phase 1/2 clinical trial of its leading product, alpha-1 antitrypsin (AAT) of human origin that can be given by infusion, for the treatment of children with diabetes newly diagnosed type 1 (T1D).
In a 1-week, open-label phase 2/37 study, children with newly diagnosed type 1 diabetes (T1D) were tested. The study involved 24 patients who received 18 treatments of 40, 60, or 80 mg/kg/dose of AAT over a period of 28 weeks. The main indicators in the trial were safety and tolerability. Secondary measures included glycemic control, C-peptide, and antibody levels. Possible responders to treatment were defined as patients with C-peptide levels that showed a decrease of less than 7.5% from baseline.
No significant side effects, cases of ketoacidosis (DKA), or severe hypoglycemic conditions were reported. The side effects were not related to the dose and were transient. Measures related to glycemic balance improved during the study in all groups, regardless of dose. Glycated hemoglobin (HbA1c) decreased from 8.43% to 7.09% (mean, p < 0.001). At the end of the study, 18 subjects (75%) had peak values of C-peptide ≥0.2 pmol/mL. Eight patients (33.3%) were considered potential responders and were characterized by a shorter period of juvenile diabetes when they entered the trial (54.5 ± 34.3 vs. 95.9 ± 45.7 days, p=0.036) and a greater decrease in their HbA1c levels during the study period (−2.94 ± 1.55 vs. −0.95 ± 1.83%, p=0.016).
In the paper, Rahmiel et al concluded that “AAT treatment was safe and well tolerated in children with newly diagnosed autoimmune diabetes. It should be noted that this is the first study that demonstrates high safety in the use of different doses of AAT administered by infusion to children who do not suffer from the alpha-1 genetic deficiency disease (AATD) and who suffer from newly diagnosed type 1 diabetes, while maintaining glycemic balance and a smaller decrease in C-peptide levels.
"We are pleased with these positive data published in the journal Pediatric Diabetes as they validate results from previous studies showing that AAT treatment can reduce pro-inflammatory markers and may protect pancreatic cells from autoimmune reactions in newly diagnosed diabetics as measured by HbA1C and C levels -peptide", noted Amir London, CEO of Kamada. "We continue to recruit patients for our phase 2/3 trial of AAT treatment in newly diagnosed type 1 diabetes patients and look forward to progress in this important trial, which we believe may change the way type 1 diabetes patients are treated."

One response
I liked it, the system that simulates bones is an excellent system, there is room for improvement and utilization. excellent