Is it possible to hold a perfectly round drop of water on flat surfaces, even when those surfaces are vertical or upside down? In the nanometer world this is possible
Is it possible to hold a perfectly round drop of water on flat surfaces, even when those surfaces are vertical or upside down? A team of scientists at the Weizmann Institute of Science designed surfaces that are able to hold a drop of water on them in almost any condition. This invention may have several applications in the fields of chemistry and biochemistry.
Surfaces that hold water are called hydrophilic or "water-loving". Because of this affection, the water droplets that stick to them become flattened, so that the size of the contact area between the water and the surface expands. On the other hand, in the face of a hydrophobic, "water-hating" surface, the water drops remain round like beads, but they roll off the surface if it is placed on any slope or as a result of a slight gust of wind. Prof. Ron Naaman, Dr. Adam Winkleman, and research student Gilad Gutsman, from the Department of Chemical Physics at the Weizmann Institute of Science, together with Dr. Alexander Yufa from the Department of Chemical Research Infrastructures, used nanoengineering methods to create a surface that maintains its round shape of the drops - but holding them in place.

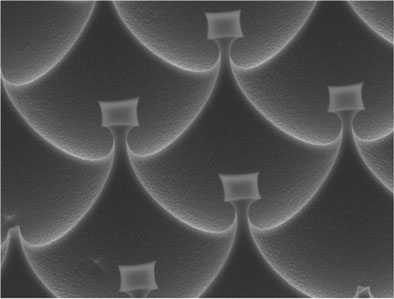
The secret of the new surfaces lies in the fact that they are hydrophilic and hydrophobic at the same time: in normal dimensions of millimeters or centimeters they are hydrophobic - the attraction between them and the water is very weak. When you look at them in dimensions of microns (millionth of a meter), the surface looks like an egg box, full of depressions the size of a red blood cell, and in between smaller bumps, the size of a few nanometers (a nanometer is a billionth of a meter). The scientists don't understand why this surface structure is so successful at holding water droplets, but they hypothesize that tiny bubbles of air trapped in the depressions are what hold the droplets.
Prof. Naaman and the members of the research group he heads created their surface from a familiar material - silicon - using processing techniques and chemical processes that are widely used in the electronic chip industry, and coated the surface with a layer of organic molecules. Capturing water droplets on a surface may be a convenient way to study them, and allow, for example, the testing of small amounts of pollutants. In addition, tiny droplets that are fixed in place may advance the possibilities of studying different single molecules, such as proteins.

4 תגובות
A. Ben-Ner 🙂 You killed me
giving:
Quote from the article:
"Surfaces that hold water are called hydrophilic or "water-loving".
This is the connection to love
I don't want to sound petty, but what's the deal with the title "because of love"?
The picture shows three grown men,
outside the building. Quite satisfied that they succeeded
To place a tiny drop of water on a "silicone surface" with an "organic substrate".
The drop, by the way, probably stayed inside.
A rather sterile description of the drama that took place there.