As you remember, scientists managed to design and produce an artificial enzyme, which also underwent "evolution in vitro". Researchers from the Weizmann Institute and the University of Washington in Seattle are partners in the project
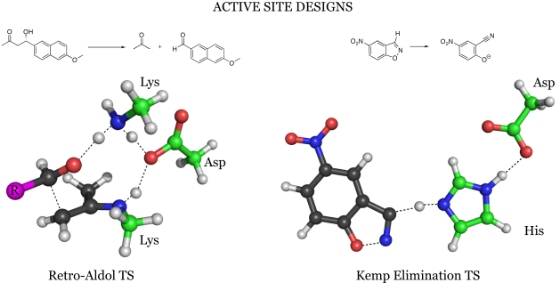
An enzyme is a biological catalyst. It is a protein molecule made of a "string" of amino acids, which fold to form a defined three-dimensional structure. The team of researchers approached, in the first step, to design the core of the machine - the "active site" - where the chemical reaction takes place. The role that the scientists attributed to the new enzyme is to remove a proton (a positively charged hydrogen atom) from a carbon atom. To this end, they created an array that includes an amino acid whose role is to "pick up" the proton, and additional amino acids that speed up the process of transferring the proton.
The next step in the process was designing the enzyme skeleton, that is, determining the sequence of the approximately 200 amino acids that make up the protein. Apparently, the number of ways to arrange amino acids from 20 different types, in a string consisting of 200 units, is almost infinite. But in fact, only a limited number of possibilities actually come into consideration, because the sequence of amino acids determines the spatial structure of the enzyme, and therefore also affects its activity. Prof. David Becker from the University of Washington in Seattle used computerized methods to scan tens of thousands of possible sequences, and located about 60 structures capable of supporting the designed active site.
Eight of them successfully passed from the computer model stage to the protein stage in vitro, and were found to be biologically active. Of these, the three most effective enzymes advanced to the "final stage". Dr. Orly Diem-Boutbol and Dr. Shira Albeck from the Department of Structural Biology at the Weizmann Institute of Science, deciphered the spatial structure of one of these enzyme molecules, and confirmed that the structure actually created is almost exactly the same as the one designed and designed using the computer software.
At this point, the efficiency of the new enzymes fell far short of the efficiency of natural enzymes perfected over millions of years of evolution. Here the ambitious research could have encountered a significant difficulty, but Prof. Dan Toufik and his research student, Olga Khersonsky from the Department of Chemical Biology at the Weizmann Institute developed a method that allows protein molecules to go through a process of accelerated evolution, mimicking natural evolution. This method is based on causing random mutations, and scanning the variety of enzymes created, with the aim of finding those that have improved their efficiency. These enzymes go through another round of mutations, and God forbid. Seven cycles of "evolution in vitro" improved the efficiency of the new enzymes 200 times compared to their original efficiency, allowing them to speed up the selected reaction a million times compared to a chemical reaction occurring without an enzyme.
Thus, for example, mutations in the shell region of the active site, caused small spatial changes in the structure of the site that corrected defects in the computerized planning of the position of the amino acids in the active site. It also turned out that the correction of tiny defects, at the level of a millionth of a millimeter, greatly affected the rate of proton passage. Other mutations increased the flexibility of the enzyme, thus helping to release the product from the active site more quickly.
"The combination of technologies - determining a structure through computerized planning, and an evolutionary process in a test tube - opens up new horizons in the production of artificial enzymes" says Prof. Toufik. "Thanks to this research, we understand more about the structure and modes of action of enzymes. This understanding will enable the design and production of enzymes for applications that nature did not "think of", such as detoxification, drug production, and many other processes."

2 תגובות
Can you post more about the method of test tube evolution?? I heard about a similar method for improving software, in which they also created mutations and selected the best ones each time.
It seems that this research is groundbreaking, and designed enzymes can be used in a variety of fields such as environmental quality, biofuels and medicines.
In addition, knowledge about the mechanisms of action of existing enzymes will greatly increase