Vulnerability. Weizmann Institute researchers deciphered the role of the accumulated mutations in the antibodies that fight the AIDS virus

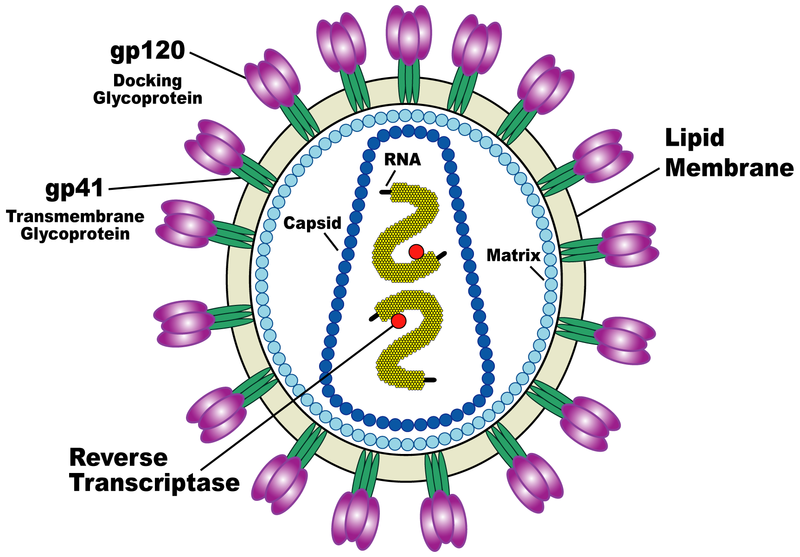
The HIV virus, which causes the AIDS disease, is an insidious enemy: it invades precisely the immune cells that are supposed to protect us from infections, slips into them precisely through their "formation of fortifications", and mobilizes the cell's mechanisms to its advantage in order to replicate. To recognize the immune cells and infect them, the virus uses protein aggregates consisting of three parts: these are spike-like structures strategically placed around the outer envelope of the virus. Dr. Ron Diskin, who recently joined the structural biology department at the institute, says that these protein aggregates have been studied extensively, and yet, surprisingly, no one has yet been able to decipher the three-dimensional structure of an entire aggregate. The reason for this is that the proteins are almost completely covered in sugar molecules with a flexible structure, which make it difficult to access the protein. One of the first goals that Dr. Diskin set for himself in his new laboratory at the Weizmann Institute was to find a way to decipher the atomic structure of the spike-like protein aggregates.
In his post-doctoral research at the California Institute of Technology, Dr. Diskin studied another aspect of infection with the AIDS virus: antibodies against the virus that some people are able to produce naturally. These are broad-spectrum antibodies capable of neutralizing a wide variety of HIV viruses, called bNAbs. Some people who produce such antibodies will not develop AIDS, even after being infected with the virus. In fact, the very existence of such antibodies is a surprising discovery: the AIDS virus is notorious for its ability to evade the immune system's antiviral mechanisms. It does this, among other things, through rapid changes in the structure of its coat proteins, caused by mutations. These structural changes mean that antibodies that are effective against one form of the virus will be useless against other forms. In contrast, broad-spectrum antibodies attack relatively conserved sites - that is, those where mutations usually do not occur - on the virus's envelope proteins, and therefore maintain their effectiveness against a wide range of forms that the virus takes.
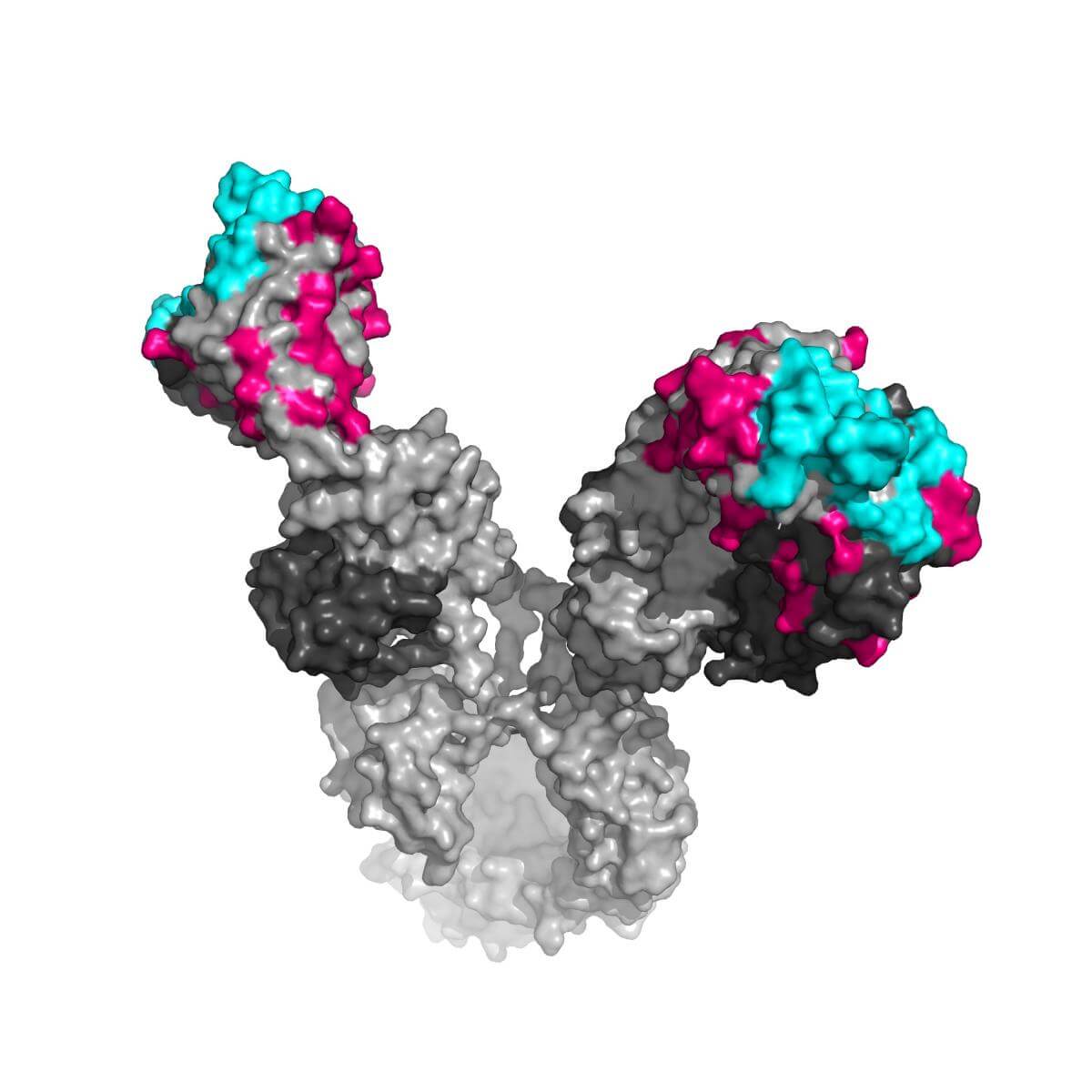
In the study, published in the scientific journal Cell, Dr. Diskin and his colleagues examined what makes these antibodies so unique. The approach taken was to decipher the role of the accumulated mutations in the antibodies. The production of antibodies occurs similar to a rapid evolutionary process: in the first stage, primary antibodies are created in which a large variety of mutations affect their shape. These antibodies undergo selection, which leaves only the antibody that best binds to the cause of the disease. Thus, when the immune system is exposed to a disease agent for an extended period of time, it continues to perfect its weapons. The researchers' attention was drawn to the fact that these antibodies contain an especially large number of mutations compared to other antibodies, and that they include surprising changes in the protein sequence in unexpected places on the surface of the antibody. While it used to be thought that mutations occur mainly in those regions on the surface of the antibody that come into direct contact with the invader (the multitude of mutations in them and their diversity gave them the name "variable regions"), broad-spectrum antibodies also contained mutations in regions considered "fixed" - those that give the antibody the its general shape and structure.
To understand the role of the unusual mutations, the researchers re-engineered the antibodies: they returned some of them to their initial state, and compared them to the "mature" antibody with the mutations. Dr. Diskin and his colleagues discovered that the mutations in the fixed regions give the antibody a significant advantage in its fight against the AIDS virus. Thus, for example, the replacement of a single amino acid in the same region in an antibody that does not bind to the cause of the disease, reduced the structural stability of the antibody, thus giving it greater flexibility. The researchers believe that this flexibility allows him to adapt to different forms of viruses.
According to Dr. Diskin, the research may have practical implications, both for the development of a vaccine against AIDS, and for the development of immune strategies against other disease agents. In addition, it reveals basic principles concerning how antibodies are formed. The scientists believe, for example, that the creation of an effective antibody, in which the correct combination of such a large number of mutations, may take several years. Therefore, even the lucky people who produce broad-spectrum antibodies will only be able to create effective antibodies for a long time after being infected with the virus. In addition to this, the discovery that significant mutations may also occur in remote areas from those that scientists have focused on so far, which disproves the accepted theory, reveals new aspects in the production processes of antibodies and their mode of action, and therefore may be relevant for the development of vaccines against additional disease agents.
Dr. Diskin is a crystallographer, that is, he reveals the three-dimensional structure of proteins by forming them into crystals, irradiating them with X-rays, and creating a structural model of them based on the way the rays scatter when they are returned from the crystal. In addition to revealing the structure of the protein clusters on the envelope of the AIDS virus, in his laboratory at the Weizmann Institute he plans to study other proteins that come into contact with viruses, interactions between proteins, as well as how individual proteins are organized to form large and complex structures.
personal
Ron Diskin was born and raised in Jerusalem, where, at the Hebrew University, he also acquired his scientific education: bachelor's and master's degrees in life sciences and biochemistry, and a third degree in structural biology in the group of Prof. Oded Livna. He then embarked on post-doctoral research at the California Institute of Technology. In 2012, he joined the faculty of the structural biology department at the institute. From his childhood he was attracted to nature, and also liked to take things apart to know how they work. This combination led to choosing a specialization in structural biology, which deals with the structure of molecules as a basis for understanding how they work.

One response
I discovered your site about a week ago and I really enjoy it
Thank you!