Recently, a new type of hydrogen bonds was discovered between the boron-hydrogen group and the pi electron array of the benzene ring. This non-classical hydrogen bond can be detected in the gaseous state of the compound formed between diborane and benzene

[Translation by Dr. Nachmani Moshe]
Recently, a new type of hydrogen bonds was discovered between the boron-hydrogen group and the pi electron array of the benzene ring. This non-classical hydrogen bond can be detected in the gaseous state of the compound formed between diborane and benzene.
Researchers Dieter Cremer and Wenli Zou from the Department of Computational and Theoretical Chemistry at Southern Methodist University in Dallas, USA worked in collaboration with chemists from Nanjing University in China in order to explore the theory of non-classical hydrogen bonds that may form between the boron-hydrogen (B–H) group. and organic structures and demonstrate the activity of such a system experimentally.
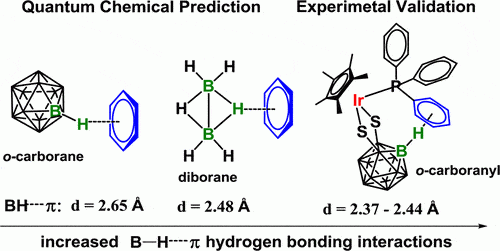
Non-covalent bonds between aromatic rings and hydrogen bonds to carbon, nitrogen or oxygen atoms are essential in molecular biology. These weak electrostatic bonds are the basis for a multitude of biomolecular interactions between proteins, nucleic acids and the other biological molecules on which living beings are based. Chemists working in the applied and theoretical fields have examined these relationships in detail and developed computer models of benzene-haloalkane, benzene-ammonia and benzene-water systems. Bonds of this type, found, for example, in systems such as Carborane (Wikipedia), have given rise to an innovative approach to designing drugs in which a boron-hydrogen group reacts with hydrogen-donating groups present in the target biomolecules. However, to date no interactions between a boron-hydrogen group and an aromatic ring have been observed.
The research team points out that in light of the known behavior of such systems, we should have expected that the electropositive nature of the boron atom should have caused repulsion rather than attraction between a boron-hydrogen compound and an aromatic ring. At the same time, the unique bond in the diborane compound itself is unusual, that is, three atoms connected by only two electrons. The research team hypothesized that the boron-hydrogen group in the diborane system may be stabilized by the slightly positively charged hydrogen atom. The research team's quantum calculations indicated that just such a bond might exist and that the interactions would indeed be electrostatic. The researchers created the non-classical bond between diborane and an aryl group within a phosphine derivative based on an iridium-dimercapto-carborane conjugate. An observation obtained with the help of X-ray diffraction revealed that the distance between the hydrogen atom and the Pi system of the aromatic ring is 2.76-2.40 angstroms, close to predictions. The researchers note that these couplings seem to form systems of oligomers, so the next step in their research will be to examine the possibility of obtaining monomers. In addition, the researchers also plan to examine the possibility of making this connection serve as an "on-off" switching system.