Researchers have developed a new reaction that converts chiral molecules into stereoisomers that are opposite in direction. The reaction focuses on inactive stereocenters that until now have been challenging to react
[Translation by Dr. Moshe Nachmani]
The innovative reaction could allow researchers to produce new molecular configurations and change the way chemists plan the synthesis of molecules. When chemists plan the development of chiral molecules they tend to use reaction sequences that focus on building chiral centers. This part of the synthesis is the most challenging part during a synthetic project, says Alison Wendlandt of the Massachusetts Institute of Technology in the US.
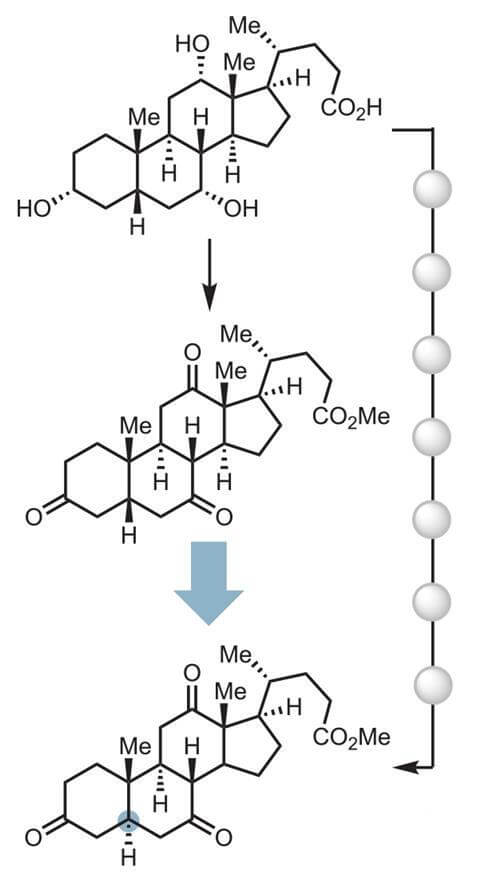
Now, her research team has found a method to produce diastereomers of complex drug-like molecules through a completely different strategic process. "Our tool actually allows us to adjust the three-dimensional structure in the later stages of the synthesis," explained the lead researcher. "Thus, if a chemist has one stereogenic center, it is now possible to produce a completely different diastereomer without going back and resynthesizing the molecule with the desired stereometric configuration." That is, when planning a synthesis, chemists can now control the three-dimensional configuration through the creation of desired carbon-carbon bonds, explains the lead researcher.
The innovative method is based on a tungsten polyanionic photocatalyst and disulfide cocatalysts that encourage an epimerization reaction, a reaction during which the configuration of a chiral center is reversed. This research team was able to reverse the stereochemistry of chiral centers in sugars in the past, and has now demonstrated in their latest research that the reaction works on a wider scale even in molecules containing inactive stereocenters of carbon atoms.
"I really believe that this reaction can help a lot in the applications of medicinal chemistry," the researcher stated. "It is very difficult to predict what the effect of a certain stereocenter will be on the interactions with, for example, an enzyme or protein - and then see what the final molecule will actually do."
Using this approach of stereogenic editing will allow drug discovery chemists to simply synthesize the easier-to-produce diastereomer and then easily convert it to the required reverse isomer. "Even if you got a ten or twenty percent recovery of the desired product, that's enough for pharmaceutical research: is this a better candidate to be used as a drug? Or is the molecule less good? And it can save you a lot of time," explains the lead researcher.
One of the world's greatest experts on photocatalysts points out that although there are several approaches to make the chiral centers near polar functional groups, "however, the ability to change the stereochemistry of an inactive carbon in a complex molecule is truly extraordinary." "I believe that the new reaction can affect the field of synthetic chemistry in many ways, but I am most excited about the way it will affect very well-established stereochemical reactions, such as the Diels-Alder reaction [formation of rings from non-cyclic starting materials], which would otherwise be particularly challenging." The fact that the reaction utilizes common and established reactants simply makes this invention even more impressive and easy to implement."
Article Summary The news about the research
More of the topic in Hayadan:
