A day after Prof. Dan Toufik's death, his hypothesis about the origin of life was significantly strengthened
Two biological mysteries, separated by a gap of billions of years, met in research held recently at the Weizmann Institute of Science.
The first mystery is the greatest of all - the origin of life. More precisely, the origin of proteins - those biological molecules that are essential for the existence of life as we know it. How were these complex and sophisticated molecular machines created from the short protein segments that were expected in the "primitive soup" - the primordial and mineral-rich aqueous environment that prevailed, according to the hypotheses, on the earth about four billion years ago?

To answer this question, scientists in the laboratory of Prof. Dan Toufik The deceased in the department of biomolecular sciences of the institute, along with their research partners, a mathematical model aimed at reconstructing the lost ancestor of modern proteins. According to the model they created, the ancestor consisted of two short protein segments called peptides, which had an almost identical sequence of amino acids - the building blocks of proteins. In other words, the ancient protein was composed of a molecular "word" that repeated itself twice and created a kind of ancient molecular "sentence".
To reproduce the process of the formation of the protein in the laboratory, the scientists created the The putative ancestor theirs and then cut it in two - a peptide and another peptide - and put them in an aqueous environment, with the aim of seeing what would happen and whether the "words" would connect to a "sentence". In the first stage, no signs of connections were observed, but when Dr Liam Longo, then a post-doctoral researcher in Prof. Toufik's laboratory, added RNA to the solution - molecules that were hypothesized to be common in the ancient soup - the water suddenly became cloudy. With the help of a microscope, Dr. Longo discovered that the peptides, which have a positive charge, were bound to the RNA molecules, which have a negative charge, and in the aqueous liquid, droplets were formed in the process of separating phases - similar to fat droplets in chicken soup.
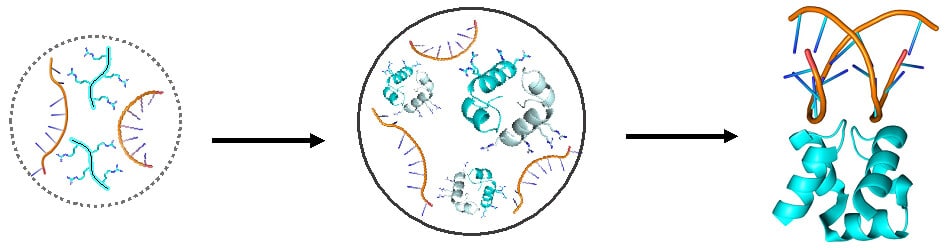
"It was an exciting discovery because the existence of such droplets in the ancient soup could have increased the chance of connections between peptides and the creation of the progenitor of the proteins, as predicted in the model," recalls Dr. Longo, who currently heads his own research group at the Tokyo Institute of Technology.
The hunt for dimmers
A small jump in time - from the era before the appearance of life on Earth straight to the beginning of the 21st century. About a decade ago, it was discovered that drops similar to those that were supposedly formed in the ancient soup, are formed naturally in the cells of our body and are even composed of proteins or a combination of proteins and RNA or DNA molecules. In 2018, the drops starred in the list of candidates for the title "The scientific breakthrough of the year” of the scientific journal Science And they were crowned "one of the hottest topics in cell biology". Indeed, as far as the droplets are concerned, there is still much that is hidden over the visible: for example, it is not yet known if they are used only for storage or if they have control functions in the cell.
It was precisely at this point that the paths of the two biological mysteries crossed: Prof. Toufik set out to investigate the potential role of the droplets in the formation of ancient proteins, and at this point it was clear that the findings were expected to shed new light also on the effect of the droplets on human health today.

Prof. Tawfik and his colleagues hypothesized that the droplets formed in the ancient soup following meetings between peptides and RNA molecules served as a sort of primary cells, proto-cells, which may later have developed into the living cells we know today. According to the hypothesis, the droplets created closed and separate spaces that allowed for close and connection Between peptides that otherwise might not have found each other in the vastness of the ancient soup. This proximity could allow the peptides to organize into simple chemical structures called dimers: pairs of peptides attracted to each other by molecular forces. In the next step the dimer fused into a single molecule, thus creating the same ancestor that the scientists had predicted.
To confirm this hypothesis, the scientists first had to find evidence for the existence of dimers in the same peptide solution they created, but the standard methods did not allow them to reach a high enough resolution. To overcome this problem, Prof. Toufik and his group collaborated with Prof. Daniela Goldfarb from the department of chemical and biological physics of the institute, which studies proteins using electronic paramagnetic resonance.
"This collaboration resulted from my friendship with Danny," recalls Prof. Goldfarb of Prof. Toufik's appeal. "He told me that he had a theory about the evolution of proteins, and I offered to test it with the help of my laboratory's capabilities." Prof. Goldfarb and her group began to search for Prof. Toufik's putative dimers using innovative methods: they labeled peptides using electronic spin tags - tiny molecules with unique magnetic properties that were prepared in Prof. Toufik's laboratory Norman Matance at the Hebrew University in Jerusalem. When the tagged peptides were placed in a magnetic field, the tags became "footprints" that convey information about the properties of the peptides to which they are attached. Thus, using an imaging method known as "double electron-electron resonance" (DEER), the scientists were able to determine the distance between one peptide and another according to the strength of the magnetic interactions between the tags; A large proximity between two peptides actually indicated the formation of a dimer.
This experimental set-up was particularly challenging, and in the first measurements the researchers were unable to detect dimers at all. In the middle of the search, a disaster happened. On May 4, 2021, Prof. Toufik fell to his death in a mountain climbing accident. As fate would have it, the very next day, Dr. Manas Sil, a post-doctoral researcher in Prof. Goldfarb's laboratory, found the first dimers in a solution of peptides. "It breaks my heart that Danny didn't get to see this find," says Dr. Longo. "He so wanted to see these dimers".

The next step was to show that the dimers are formed not only in a solution of peptides, but also inside the droplets themselves - when the peptides are attached to RNA molecules. First, the scientists showed that the peptides did bind to the RNA inside the droplets, through observations that showed a slowdown in the movement of the spy tags. However, the next step - the creation of the dimers - was difficult to show due to the great crowding inside the droplets. In the end, after testing different concentrations of RNA and peptides in the droplets, the scientists were able to prove that dimers are also formed in the contents.
"Basically, we showed that binding to RNA molecules increases the formation of dimers," says Dr. Seal, who led the experiments. "This link probably helps the peptides find each other, like two people holding the ends of the same rope."
These findings greatly strengthen Prof. Tawfik's hypothesis, according to which the droplets formed in the ancient soup promoted a crucial stage in the evolution of proteins - the creation of dimers from blue-chain peptide pairs on the way to creating longer proteins - and therefore contributed to the appearance of life on Earth. "'Cradle of the evolution of proteins', that's what we called the drops in our article," notes Dr. Longo.
Besides a better understanding of the beginning of life, these new insights may also contribute to the understanding of human health in the future. For example, according to different opinions, defects in the formation of the droplets in our cells may lead to the development of degenerative brain diseases. Therefore, a deeper understanding of the droplet formation mechanisms and their functions may pave the way for new diagnostic methods and treatments.
Dr. Dragana Despotovic from the late Prof. Toufik's laboratory also participated in the study; Orit Weil-Katorza and Prof. Norman Matans from the Hebrew University of Jerusalem; and Prof. Yaakov Levy from the department of chemical and structural biology of the institute.
