Researchers at the Technion and the Yulich Institute in Germany developed a titanium-air battery and demonstrated its effectiveness experimentally
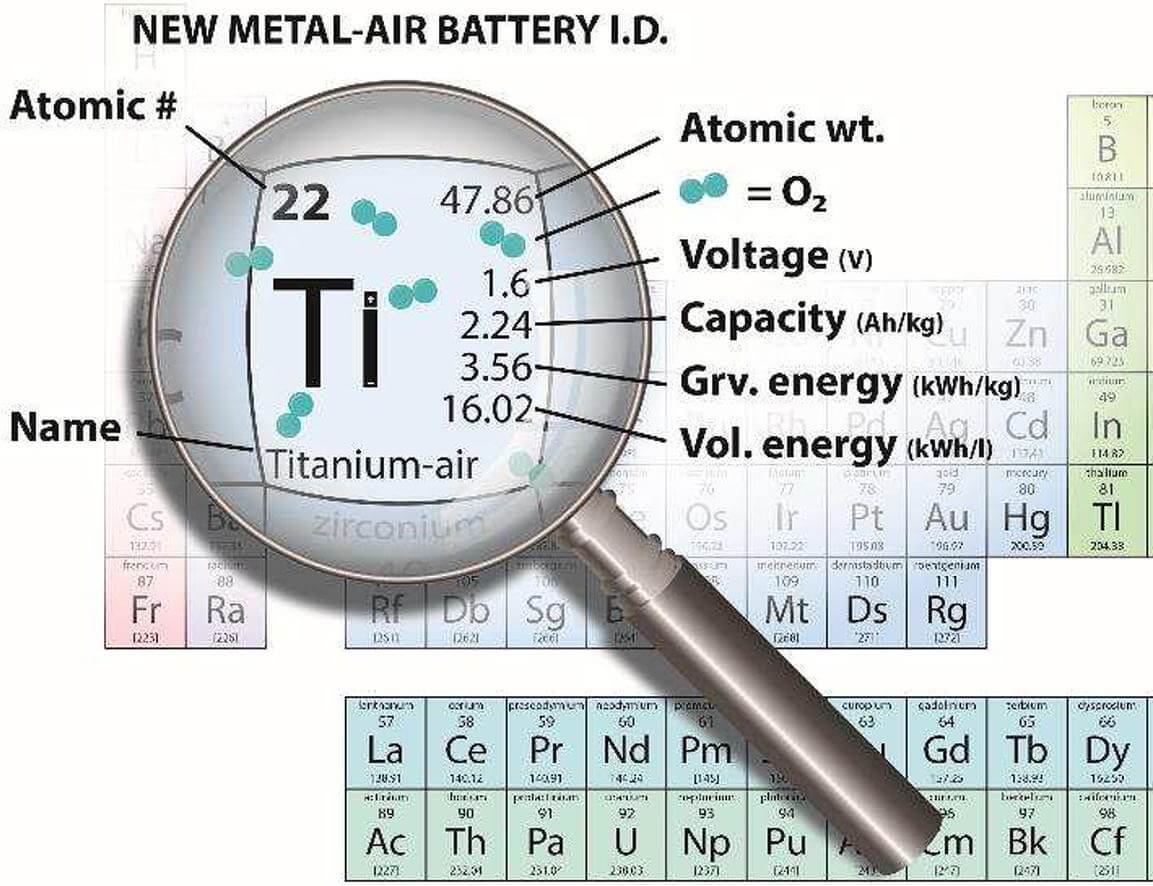
Researchers at the Technion and the Yulich Institute (Germany) developed an innovative titanium-air battery, and verified its effectiveness experimentally. The development of the new battery was made possible thanks to the collaboration between Dr. Yassin Amri Dormos, from the Yolich Institute for Energy and Climate Research (No. 9) led by Prof. Rudiger Eichel and Prof. Yair Ein-Eli from the Faculty of Materials Science and Engineering at the Technion. The article by Technion researchers and Yolich was published in the journal Chemical Engineering Journal, and this is the first publication of experimental findings regarding this type of battery, in which titanium is used as an active material.
Batteries are devices that convert chemical energy into electrical energy using an electrolyte (the material that conducts ions) and two electrodes immersed in it. One of the electrodes (the one with very low voltage) removes electrons in the external circuit (which activates the electronic instrument), and the other electrode receives the electrons. The electrochemical process takes place within the electrolytic medium that contains ions, and this is how the total electric current needed to operate the electronic equipment and to discharge the cell (battery) is created.
A metal-air battery is a family of batteries in which one of the electrodes is made of an active metal (with very low voltage), and the other electrode is a thin membrane, which allows the entry and reaction of air, and more precisely oxygen. Since the oxygen comes from the surrounding air there is no need to store it, thus actually saving valuable space (volume) and weight inside the battery. Therefore, the energy content of this type of battery should theoretically be higher compared to other batteries. Metal-air batteries are particularly suitable for applications that require miniaturization as well as for large storage systems where cheap, readily available, and non-toxic materials are required.
If so, what is the optimal metal for metal-air batteries? To date, mainly lithium, zinc, iron, aluminum and silicon have been studied. Zinc-air batteries, for example, are already used in control devices, sensors and hearing aids. However, these batteries are characterized by relatively low energy density for the growing needs of the energy market, and therefore they provide energy for a relatively short time.
Titanium-air batteries, the effectiveness of which was demonstrated in the present study, are characterized by an energy density twice as high as that of zinc-air batteries, and therefore they are expected to provide energy content for twice as long and more. It should be noted that titanium is a common chemical element - it ranks ninth in the list of common materials in the earth's crust. Titanium is known as an immune, stable and extremely durable metal, which does not react at all with the environment, not even with the most aggressive reactants, and this is due to the fact that titanium is covered with an effective and very durable oxide protective layer.
The Technion researchers and Yulich were able to remove the strong protective layer of the titanium only, using a unique electrolyte in the battery they developed. The aforementioned electrolyte is a type of ionic liquid with unique characteristics - a liquid made of salts and suitable for use in batteries due to its electrical and chemical properties. This is how the researchers were able to harness the chemical potential of the exposed titanium metal for conversion into electrical energy. In their experiments they demonstrated, as mentioned, an electrical energy content hundreds of percent higher than that of zinc-air batteries.
The research was supported by the German Federal Office for Education and Research, and the Technion researchers were supported by the Grand Batechnion Energy Program (GTEP) and by the Research Center for Electrochemical Storage and Propulsion (INREP).
Prof. Ein-Eli stayed at the Yulich Institute as part of a sabbatical year, as part of the framework agreement between the two institutions and Aachen University. The framework agreement (Umbrella) between the institutions celebrates its 40th anniversary this year, and the upcoming symposium will be held in Haifa between May 30 and June 1, 2023.
for the scientific article in Chemical Engineering Journal
For pictures click here
