Weizmann Institute of Science scientists deciphered the molecular mechanism of "return to normal" and discovered that it is based on the labeling of proteins for destruction with a special code known as "SUMO"
Dealing with stress factors is important not only for us but also for our cells. In extreme distress conditions, the cells create temporary aggregates called "stress granules" that help them deal with heat, cold or other distress situations that may harm them. However, when these granules are not broken down after the danger has passed, they can accumulate in the cabin - like old cars filling up a junkyard. These "scraps", it is believed today, may lead to degenerative diseases of the nervous system. Weizmann Institute of Science scientists, together with their research partners, The molecular mechanism was recently deciphered by which the arachnoid granules are broken down in healthy cells and showed that strengthening this mechanism may inhibit neurodegeneration characteristic of amyotrophic lateral sclerosis (ALS).
Eka granules consist of RNA molecules and proteins that bind to them. It is now known how these granules are formed, but until now it was not known how they break down. Damaged Eka granules that have not broken down properly are considered a preliminary stage for the formation of protein clusters called inclusion bodies and are found in the brains of those affected by ALS, frontal lobe and temporal lobe dementia, and other degenerative diseases. Prof.'s group Eran Hornstein from the department of molecular genetics of the institute investigates the process of breaking down these granules in order to understand what happens when this process goes wrong.

Research students Hagi Marmor-Collet and Aviad Siani from Prof. Hornstein's laboratory led the research, which was conducted in collaboration with Prof. Tami Giger from Tel Aviv University and with researchers from Harvard University Medical School and the University of Leuven in Belgium. The scientists developed new research methods that allowed them to isolate very small sections of human cells and characterize all the proteins, using mass spectroscopy. This is how the scientists mapped the contents of the aka granules and identified about a hundred new proteins and microscopic substructures that were not known until now. To their surprise, they discovered that while the granules undergo decomposition, they recruit dozens of additional proteins, which are directly involved in the decomposition processes.
The scientists succeeded in deciphering a new and surprising chain of biochemical reactions for breaking down the acacia grains, which is based on the labeling of proteins for destruction with a special code called "SUMO" (SUMO - acronym for small ubiquitin-like modifiers). Thus, the researchers were excited to discover that such a specific activity plays a vital and unexpected role in the granulation process.
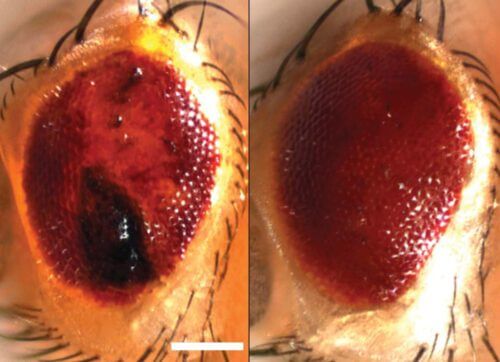
The case of ALS disease
The scientists found that their findings were valid for degenerative brain diseases: after exposing cells to a toxic protein that causes ALS in humans, the AKA granules did not break down properly and SUMO tags were not properly recruited. Furthermore, when the scientists applied the sumo array to fruit flies that had been engineered to develop an ALS-like disease, they were able to halt the degeneration of their nerve cells.
The findings open a new research direction of the mechanisms underlying ALS and other neurodegenerative diseases. "Our research may encourage the development of future treatments, since increasing the activity of sumo tags appears to be a promising new direction for the treatment of neurodegenerative diseases," says Prof. Hornstein. Prof. Hornstein's laboratory has been specializing in ALS research for many years and has developed a treatment that is currently in the second phase of a clinical trial in ALS patients at the Montreal Neurological Institute (MNI) in Canada; Another treatment developed in the laboratory is in the pre-clinical stage. Both treatments rely on microRNA-based mechanisms - tiny biological molecules that play different control roles in the cell.
Natalie Rivkin, Yehuda-Matan Danino, Dr. Tzvia Olander, Daoud Shiban, Nir Cohen, Roital Ravid, Chen Eitan, Dr. Beata Tot Cohen and Prof. Yaakov Hana from the department of molecular genetics of the institute; Dr. Tali Dadosh from the Department of Chemical Research Infrastructures of the Institute; Dr. Yosef Addi from the Department of Life Sciences Research Infrastructures of the Institute; Dr Yifat Marble from the department of immunology of the institute; Dr. Nancy Kadersha, Sarah Hoffman, Claire Riggs, Dr. and Yuk M. Adebani, Prof. Paul Anderson and Prof. Pavel Ivanov of Harvard Medical School; Naama Knafo from Tel Aviv University; Dr. Thomas C. Moens and Prof. Ludo van den Bosch from the University of Leuven in Belgium; and Dr. Adrian Higginbottom and Dr. John Cooper-Nock from the University of Sheffield.
More on the subject on the science website:
