The embryo model developed from stem cells that were grown outside the womb to a relatively advanced stage, showing normal development of organs and tissues
In the year 6565
You won't need no husband, won't need no wife
You'll pick your son, pick your daughter too
From the bottom of a long glass tube
From the song "In the year 2525" by Zieger and Ebens, they predicted a situation where the children would be created from test tubes without parents - perhaps they meant that even living beings can be cloned from existing stem cells?
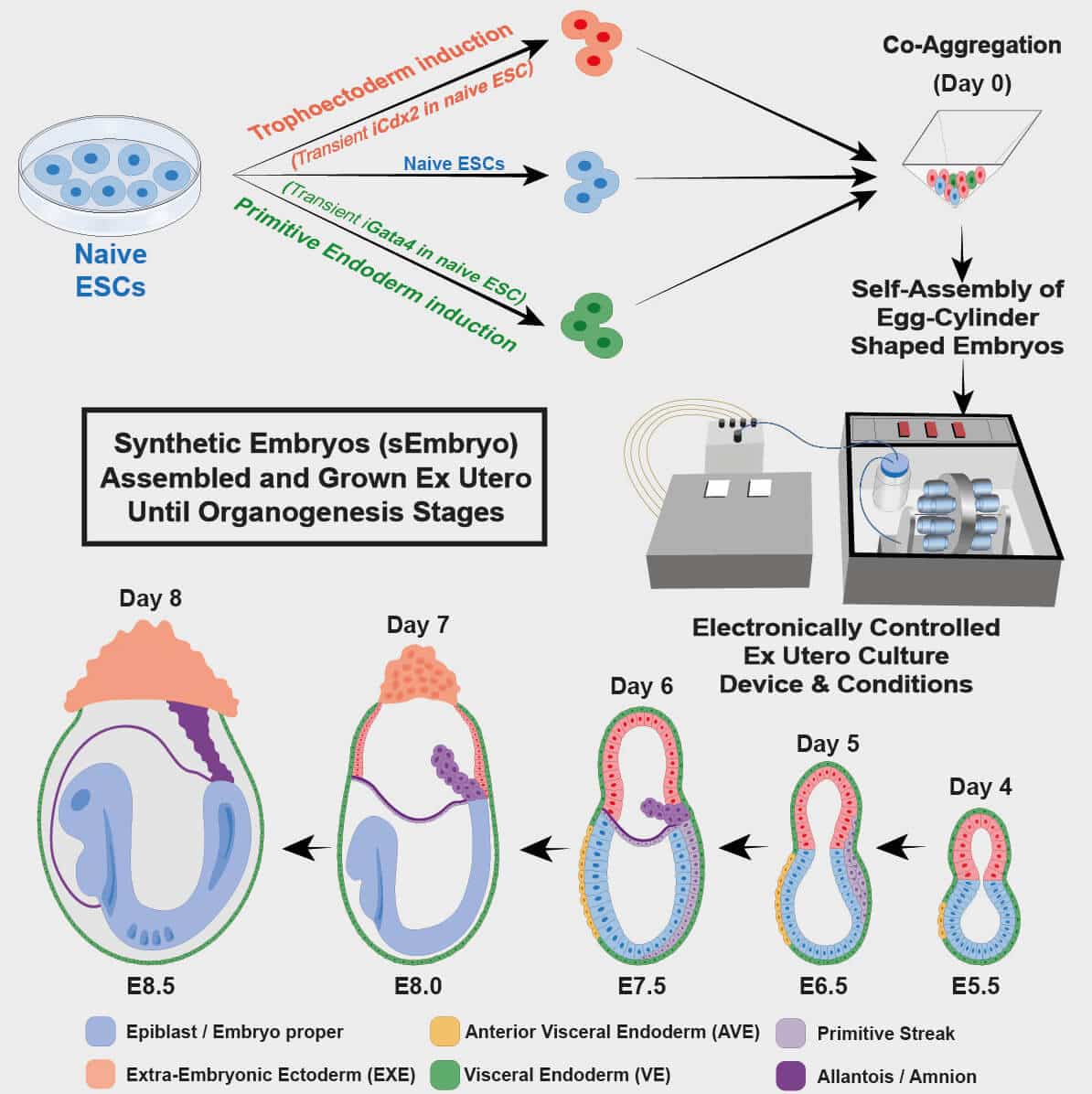
An egg meets a sperm - this is a fundamental condition for the creation of a new life. A new study by Weizmann Institute of Science scientists published today in the scientific journal Cell, challenges this basic fact of life. A research team led by Prof. Yaakov Hana created for the first time artificial models of mouse embryos that developed from stem cells and grew outside the womb - without fertilization of egg and sperm. Beyond the breakthrough in the study of embryonic development, this innovative approach may break new paths for the medicine of the future, including growing organs and tissues that can be used in life-saving transplants.

The artificial embryo model is based on two previous breakthroughs in Prof. Hana's laboratory in the Department of Molecular Genetics at the Weizmann Institute: the first, an effective method for returning embryonic stem cells to the so-called "naive" state - the most initial developmental stage in which the stem cells have the ability to differentiate not only into any type of tissue in the body of the fetus, but also to extra-embryonic tissues, namely the placenta and the yolk sac that support the development of the fetus. The second breakthrough is an innovative method for growing mouse embryos outside the womb until late stages of development. This method, which was developed over seven years of trial and error, is based, among other things, on a moving device where the embryos are held in laboratory beakers surrounded by a nutrient solution. In the absence of blood flow from the mother to the placenta, the movement of the device simulates the natural feeding of the fetus while regulating the oxygen levels and air pressure in the laboratory vessel. In a previous study published inNature In March 2021, the scientists were able to grow natural mouse embryos from day 5 to day 11 using this device.
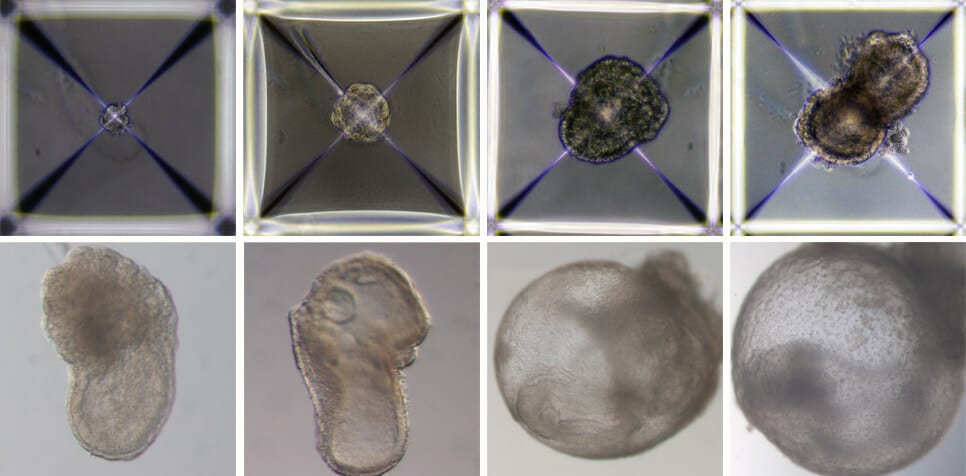
In the new study, the scientists sought to develop an artificial model of mouse embryos - that is, embryos that originate not from a fertilized egg as in the previous study, but from embryonic stem cells that have been grown for years in culture. To this end, the scientists divided the embryonic stem cells in the laboratory into three groups: one group, from which the embryo itself developed, remained unchanged, while the other two groups were treated for 48 hours in order to increase the expression of certain genes necessary for the creation of extra-embryonic tissues - the placenta in one group And the yolk sac in the second group. After the three groups were placed in the ectopic embryo-growing device, they quickly began to organize into clusters of cells, the vast majority of which did not develop properly. However, about 0.5% of them - 50 out of about 10,000 - formed spherical tissues that developed over time into a fetus-like structure.
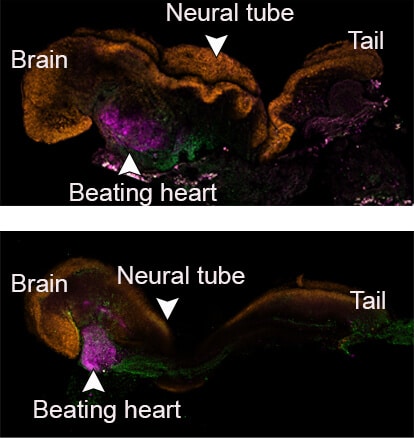
The scientists pre-marked each group of cells with a different color and thus were able to see that the artificial models that succeeded in developing did indeed develop similarly to natural embryos, and that the placenta and the yolk sac were formed separately from the embryo. These artificial models of mouse embryos developed normally for 8.5 days - almost half of the 20-day gestation period - and developed early internal organs, including a beating heart, the beginning of blood circulation, a normally shaped brain, and rudimentary versions of the central nervous system and digestive system. The developed embryos showed a 95% match to natural mouse embryos - both in the shape of the internal organs and in the gene expression patterns of different cell types.
The artificial model developed by the researchers opens new horizons in the study of embryonic development. "Our next challenge is to understand how the stem cells know what to do - how they organize themselves into organs and how they find their way in the developing embryo. In addition, since, unlike the uterus, our system is transparent, it may be used by us to study congenital and developmental defects and various fertility problems", Prof. Hana details future research directions.
Artificial models of embryos may also reduce the use of animals in research, and in the future even become a reliable source of cells, tissues and organs for transplantation. "The embryo development process is actually an elaborate mechanism for the production of organs - the best biological XNUMXD printer", says Prof. Hana. "Instead of developing a separate protocol for growing each type of cell, for example, kidney or liver cells, we may in the future be able to create an artificial embryonic model and isolate the desired cells from it. We will not dictate to the organs how they should develop - this is best done by the embryo itself".
The research was jointly led by Shadi Terzi, Alejandro Aguilera and Karin Jobran from the Institute's Department of Molecular Genetics. Also participating in the study were Shahed Ashiohi, Dr. Francesco Roncto, Emily Wildschutz, Dr. Bernardo Oldak, Alidat Gomez-Sizer, Nir Livnat, Sergey Vyukov, Dmitry Lokshtanov, Segev Noah Tessa, Max Rose and Dr. Noa Noberstern from the Department of Molecular Genetics of the institute; Montser Hadad and Prof. Zvi Lapidot from the Department of Immunology and Biological Regeneration of the Institute; Dr. Merav Kadami from the Department of Life Science Research Infrastructures of the Institute; Dr. Hadas Keren Shaul from the Israeli National Center for Personalized Medicine for Nancy and Steven Grand; and Dr. Nadir Ganem, Dr. Suhir Hana and Dr. Itai Maza from the Rambam Medical Center.
More of the topic in Hayadan:
- Weizmann Institute scientists grew mouse embryos outside the womb
- The father of the "test tube babies", Prof. Robert Edwards from Great Britain won the Nobel Prize for Medicine
- Dr. Yaakov Hana from the Weizmann Institute, one of the Krill Prize winners: The prizes give me energy to continue research
- A method for increasing the production efficiency of the stem cells
- For the first time: completely "neutral" human stem cells, which developed into tissues inside a mouse embryo

One response
So could Mary (mother of Jesus) be right in the end?