Institute scientists are updating a version of one of the most classic diagrams in biochemistry books - the Krebs circle
In a plowed field it is doubly difficult to identify a fresh furrow. The Krebs circle, or the citric acid circle as we called it in high school biology classes, is undoubtedly an example of well-plowed territory: as early as 1953, the Nobel Prize was awarded to Hans Krebs, the German-Jewish biochemist who discovered it, and since then many arrows have been added to the iconic diagram that depicts a decisive moment in the evolution of life On Earth: the transition to oxygen-based energy production. Therefore, when Dr. Lia Heineman-Yerushalmi from the laboratory of Prof. Eli Seltzer at the Weizmann Institute of Science She discovered Because there is room to update the diagram, she was surprised by the power of the discovery: "The Krebs circle adorns every biochemistry textbook, the ability to add a new arrow to such a classic diagram is a very exciting thing."
But to get to the bottom of the new discovery, one must first go back. Many years back. The first creatures on earth produced energy through a process of breaking down sugar known as glycolysis. This ancient metabolic pathway does not depend on oxygen at all and it characterizes living beings to this day - from bacteria to humans. However, throughout evolution, and with the perfection of life on Earth, the metabolic pathway of the Krebs cycle also developed, allowing for the production of a greater amount of energy and thus supporting the development and existence of complex biological life. However, even after the entry of the Krebs cycle into the evolutionary game, glycolysis remains a crucial biological role: the ability to switch between metabolic pathways when necessary allows cells to deal with different environmental conditions without going out of balance and equilibrium. Moreover, certain body tissues or developmental processes need low-oxygen conditions for their proper functioning, and therefore rely primarily on glycolysis.
In Prof. Seltzer's laboratory in the Department of Molecular Genetics, metabolic pathways are not routinely studied, but the development of bones and the skeletal system. Therefore, their initial interest in breaking down sugars arose in the context of cartilage cells - one of the body's tissues that develops under conditions of oxygen deficiency and relies on the production of energy through glycolysis. The team of researchers, led by Dr. Lital Ben Tovim, sought to understand why the cartilage tissue "gave up" the supply of oxygen and the energetic benefits inherent in it - and how low-oxygen conditions serve it.
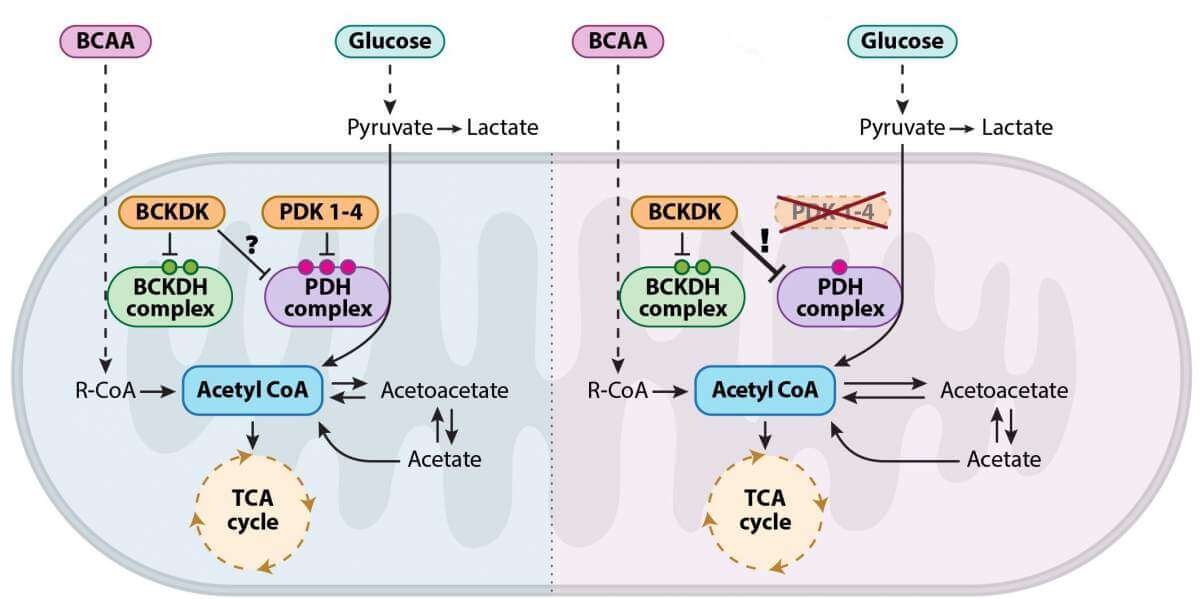
To examine these questions, the researchers tried to intervene in the cellular respiration processes in mouse embryos, to force their cartilage cells to produce energy through the Krebs cycle - and see what happens. To this end, they developed a genetic model of mouse embryos that cannot express PDK proteins - a family of enzymes responsible for regulating the transition between the metabolic pathways and the breakdown of sugar. No one expected the results obtained: despite the complete absence of the enzymes controlling the processes of cellular respiration, the embryos developed in a completely normal manner. When the researchers checked what actually happened, they saw that it was no wonder that the embryos developed properly - the control over the transition between the pathways was maintained even in the absence of the enzymes that were considered vital and irreplaceable until now.
Apparently, as far as the cartilage tissue research is concerned, the research has reached a dead end. However, Dr. Lia Heineman-Yerushalmi, then a research student in Prof. Seltzer's laboratory, could not accept the surprising findings and insisted on getting down to their metabolic roots. This determination allowed the research team to discover an unknown mechanism in a well-mapped territory: the researchers They revealed Because in the Krebs cycle there is an unknown backup plan that comes into action in the absence of the control enzymes from the PDK family: the researchers showed that the shoes of the missing enzymes are replaced by an enzyme called BCKDK, which originates from another and seemingly separate metabolic pathway - one of the pathways for breaking down proteins, or more specifically, the pathway for breaking down amino acids branched-chain.
Beyond the groundbreaking basic science, these findings may have an impact on the understanding and treatment of many diseases in which there is metabolic failure. In the last 20 years, many studies have shown that PDK enzymes are involved in cancer, metabolic diseases, such as diabetes, and neurodegenerative diseases, such as Huntington's. These findings led to the development of many different drugs designed to inhibit the activity of these enzymes, but the use of these drugs is limited due to low efficiency and the lack of a genetic model that allows a comprehensive physiological examination of their consequences. It is possible that the new findings provide an explanation for the low effectiveness of the existing drugs and open the door to the development of new treatments for these diseases.
More of the topic in Hayadan:
