study Uniquely at the Hebrew University he found that heavy water is distinctly sweeter than normal water, and may even mask a slight bitterness. "The new information will be able to help in the future in the development of new generations of sweeteners", state the researchers
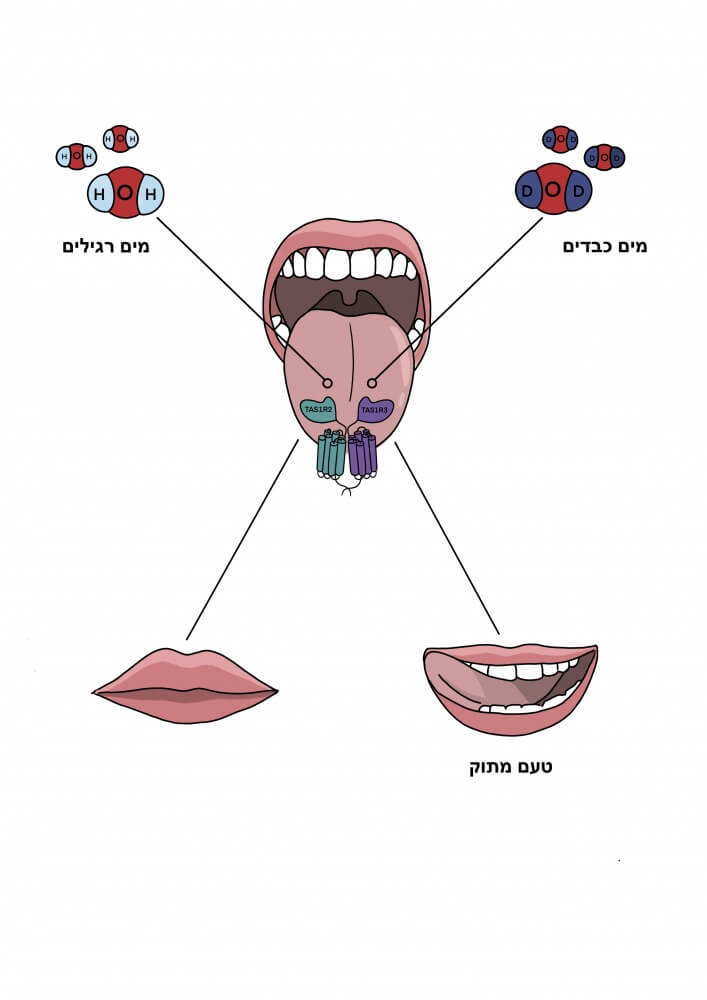
The term "heavy water" is known in Israel mainly in the context of nuclear reactors, where it is used both as a cooling agent and as a matting agent, allowing the use of uranium with a lower enrichment level. Heavy water, whose chemical formula is D2O, are actually a molecule consisting of an oxygen atom bound to two deuterium atoms (heavy hydrogen), which are found in nature in minute amounts (with a frequency of 1 to 20 million water molecules) and are produced in an expensive distillation process. They are similar in their chemical properties to ordinary water, but the hydrogen atoms that have been replaced by a heavier hydrogen isotope - the deuterium, contribute to the fact that the mass of heavy water is about 10% greater than that of ordinary water.
The heavy hydrogen was discovered by the scientist Harold Yuri, who won the Nobel Prize in Chemistry for his discovery in 1934. In 1935, Yuri and his partner published in the prestigious scientific journal Science (in response to some anecdotal news at the time about the taste of heavy water), that heavy water has the same taste for normal distilled water. This firm opinion from the Nobel laureate, in a leading scientific journal, almost shut the door on the attempt to trace the taste of heavy water. "None of us are able to detect the differences, not even the smallest, between the taste of ordinary distilled water and the taste of pure heavy water," explained Yuri and his research partner. Nevertheless, researchers continued to be interested in the taste of heavy water over the years, the matter became a bizarre phenomenon Surprising, and today you can find videos with many tasting experiments, for example those that are documented on YouTube - mainly by chemists who use heavy water in nuclear magnetic resonance (NMR) experiments.
"Of course you shouldn't drink heavy water unsupervised," hurries to warn Prof. Masha Niv from the Faculty of Agriculture, Food and Environment at the Hebrew University, who set out to investigate the long-standing mystery of the taste of heavy water with members of her lab and partners from the Czech Republic and Germany. Their results were published a few days ago in the scientific journal Communications Biology.
First it was tested in a controlled manner whether the human senses of taste and smell are able to distinguish between heavy water and normal water. 28 subjects were given three samples of water, two of them of normal water and one of heavy water. They were required the first time to smell the water, the second time to taste the water without smelling (while plugging their nose with a clip), and the third time to taste the water with the possibility of smelling it. The result was that most tasters were able to correctly identify the unusual sample based on taste (but not on smell alone). In addition, from a questionnaire they filled out, it became clear that the tasters rated the heavy water as sweeter than the normal water, and also gave a higher sweetness score as the solution contained more heavy water compared to the normal water. Also, the perceived sweetness of known sweeteners (e.g. glucose) was perceived to be higher when dissolved in heavy water compared to plain water.
Can heavy water also affect other flavors? The researchers tested the reactions of the subjects when they added heavy water and normal water to monosodium glutamate (or MSG for short, which has the appetizing umami taste) or to the substance bitter quinine (used as a medicine for malaria), and asked them to taste. "It was found that the umami flavor was not enhanced by melting MSG in heavy water. The perceived bitterness of the quinine was reduced when it was dissolved in the heavy water compared to the normal, in accordance with the known effect of sweeteners as bitterness reducers", explained Prof. Niv.
Is it possible that the receptor on the tongue responsible for sensing sugars (TAS1R2/TAS1R3) is the one that is activated with the help of the heavy water, despite differences in the chemical structure of sugars compared to the D2O? This question was tested with the help of a known inhibitor of the sugar receptor called lactisol (prevents the transmission of the "sweet" message by the receptor). If the sweetness of the heavy water is mediated by the receptor, lactisol should block it. Indeed, in a taste perception experiment among a panel of tasters, it became clear that the sweetness decreased significantly in the presence of lactisol. That is, the receptor for the sweetness of sugar is the one that is also responsible for the sweetness of heavy water.
"There are many factors that affect our sense of taste - the composition of saliva, the state of hunger and the taster's mood, and more. Therefore, testing on humans is not enough," explains doctoral student Nathalie Ben Abo from Prof. Niv's laboratory. "With the help of the laboratory director Dr. Einav Malach, I set up an experiment with cells grown in the laboratory. In the experiment we used cells that are in a plate (plate) through which we were able to directly test what happens to the receptor without a human environment, and how the cells with the receptor react to heavy water. The results of the experiment came back and supported the findings of the sensory experiments."
Although the heavy water will not become a new drink due to its involvement in many processes in the human body and its possible toxicity (unless a significant percentage of the water in the body is replaced by heavy water), and also due to its high price. On the other hand, the research states that the heavy water causes sweetness on its own, and also adds to the sweetness of sugars and therefore illuminates a new and unfamiliar facet in the function of the sweet taste receptor. "The current research resolves a long-standing controversy regarding the taste of heavy water, and the new information discovered in it could help in deciphering the transfer of singlet by receptors, and perhaps in the future also in the development of new generations of sweeteners", the researchers conclude.
More of the topic in Hayadan:
- Fabrication of cotton candy-inspired nanofibers
- Many find it difficult to swallow medicines because of their repulsive taste - will a new method make them friendlier to humans?
- A food additive will allow reducing the amounts of sugar and salt in food
- Researchers from the Hebrew University have developed an algorithm that shortens the search for healthy sweeteners
- A study published in Nature in which a MTA researcher is involved reveals a process 8 times more powerful than the fusion of hydrogen in an atomic bomb
