Researchers have traced for the first time a unique process in the growth of tiny crystals, which could improve our lives and which may have occurred at the beginning of life
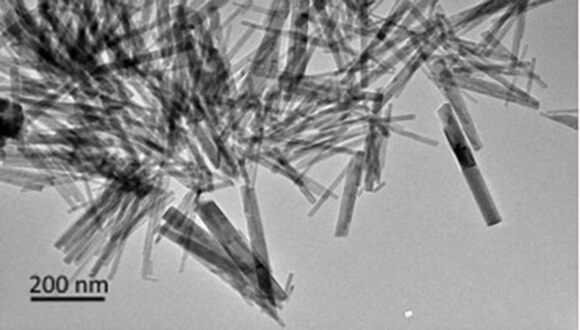
What does a drug that works well on our body have in common, to improve the quality of our computer or smartphone screen and even to use the unique properties of animals for the purpose of upgrading the existing nanotechnology? The answer: understanding the crystallization processes of crystalline structures. The nanocrystals are a microscopic configuration of particles (metals, salts, exotic elements or organic substances), which tend to organize themselves in regular geometric structures such as cubes, rods, spheres and more. A new study led by researchers from Tel Aviv University proved for the first time that there is an isotopic effect in the formation of nano-crystals which means a change in the mass of the atoms in a chemical reaction without changing their chemical nature. The researchers were able to observe the phenomenon in nanocrystals that contain phosphorus and elements from the lanthanide family, and claim that this finding can form a basis for research into other crystal systems.
The secret to understanding the formation of life
The research was led by Prof. Gil Markovich and PhD student Gal Schurtz from the school of chemistry at the Faculty of Exact Sciences by Raymond and Burley Sackler. Prof. Markovich's group investigates unique physical and chemical phenomena on the nanometer scale, both as basic science and as a step on the way to upgrading various technological devices. Prof. Amir Goldbort and Dr. Uri Hananal from Tel Aviv University, and Dr. Liat Avraham from the Department of Chemical Research Infrastructures at the Weizmann Institute of Science also took part in the research. The article was published in the prestigious Journal of American Chemical Society (JACS).
"In the world of crystals there are many different forms of symmetry according to which the atoms arrange themselves in the crystal. In quartz for example, which is the main component of sand and contains atoms of silicon and oxygen, the atoms have a helical arrangement. Such a coil can rotate clockwise or counterclockwise, and these are actually two opposite symmetries that are a mirror image of each other. Therefore, the sand we know has two populations of quartz crystals: 50% with atoms arranged in right-handed coils and 50% with left-handed coils (clockwise/counterclockwise). The 50:50 ratio is because there is usually no preference for a particular direction. We are investigating the creation of other crystals with the same type of symmetry and influencing the ratio between right-handed and left-handed. That is, they cause a kind of symmetry breaking in their formation in all kinds of ways", explains Prof. Markovich.
"Basic scientific questions like symmetry breaking in chemical processes (including formation) are also related to understanding the formation of life on Earth. The various molecules of life, for example DNA, proteins, sugars and more, are also accompanied by symmetry breaking. Therefore, the general interest of the world of research on crystal formation affects many areas of our lives, starting with the production of medicinal substances, because the form in which our body receives the medicinal substances is very important for its behavior upon entering the body. Therefore, a significant part of the work in the pharmaceutical industry deals with formulation, taking care of the form in which they will be introduced into the body, and this is done, for example, by forming the molecules into small crystals of a certain size and crystal structure," he elaborates.
"There is also the issue of biomineralization: many animals produce complex crystalline structures within their bodies such as a skeleton, armor, and the like. Understanding their crystallization processes can contribute a lot to the biological understanding of animals and also how to copy from nature the ability to create materials with special properties, such as strength, flexibility and more" Prof. Markovich provides another example.
On the way to crystallization, the effect of the chemical solvent is checked
In their new study, Prof. Markovich and his student tried to trace the effect of the type of solvent in which the nanocrystals develop, on their growth rate, and for that they compared normal water and heavy water, in which the hydrogen is replaced by an isotope called deuterium.
"When you put the components of the studied crystal into an acidic aqueous environment, they crystallize into nanorods. In the experiment, we monitored the appearance of light emission from the nanocrystals after irradiating them with ultraviolet light, a phenomenon that only occurs when the crystals begin to form. The innovation we showed is that the type of solvent does affect the growth process of the crystals. We compared a heavy water type solvent and normal water, and noticed that in heavy water the crystallization process takes a much longer time compared to normal water. The next step was to decipher the kinetic mechanism underlying this isotopic effect," explains Prof. Markovich.
Phenomena of this type, the researchers explain, help to better understand the initial processes that cause the formation of the crystals in the solution. In this study, as well as in many studies in the nano field, the researchers used advanced spectroscopic techniques for a deeper understanding of the phenomenon.
"In an attempt to continue deciphering the growth mechanism of the crystals, we used nuclear magnetic resonance measurements (the method that is the basis of the MRI), of the phosphorus atoms, and this in order to follow the preliminary stage for the formation of the nuclei of the formation. We took samples from different stages of the tumor and stopped the reaction. Thus, we found that before the formation of the primary crystal nuclei, unordered clusters of the building blocks of the crystal are formed, and when they reach a certain critical size, they immediately become the primary nucleus from which the crystal will continue to grow. It's fascinating to see that a small difference in a seemingly trivial component, the chemical solvent, affects in such a dramatic way the chemical dynamics that the particles go through on the way to crystallization," says doctoral student Gal Shortz.
Today, most of the electronic devices we are familiar with make use of nanotechnology, from TV screens to smartphones to computer processors. According to the researchers, the ability to move forward with regard to the miniaturization of electronic components, to improve their conduction and, as a result, to upgrade the user experience - is based on chemical understanding and basic science of this kind. According to the researchers, the immediate result of this study and similar ones is a better understanding of formation processes, in the laboratory and in nature, and indirectly there may also be an effect on technological materials, which in many cases also consist of crystals.
"This is an important discovery for the world of chemistry and materials science. We learned another lesson in understanding the formation processes of crystals, a topic that has been studied for tens and hundreds of years, and I hope that our research will shed light on various fields, from geology and mineralogy to nanotechnology and hardware engineering", Prof. Markovich concludes.
