The institute's scientists developed an innovative method for examining hundreds of thousands of cartilage cells in XNUMXD - and with it revealed new information about bone growth
"If you don't eat, you won't grow", children are told, but our height in adulthood is largely determined by the growth plates: growth areas at both ends of the long bones in the body, for example the bones of the arms and legs. During childhood and adolescence, the soft cartilage tissue in these areas grows and is gradually replaced by hard bone tissue. "If the cartilage is not normal, the bone will not be normal either - too short, too long or distorted," explains Prof. Eli Seltzer from the Department of Molecular Genetics, whose laboratory specializes in the study of the skeletal system. Recently, a new imaging method was developed in his laboratory that allows examining growth processes in XNUMXD. with the help of the method revealed the researchers New findings on bone growth and how this growth goes wrong in a certain type of dwarfism.
About a century ago, scientists realized that the cartilage cells are the key to understanding the growth processes, but until recently there were no effective methods for monitoring the processes that these cells go through. The existing imaging methods made it possible to produce only two-dimensional images, so the information on the volume of the cells and their organization in space was only partial. High-resolution imaging methods, on the other hand, provided detailed images, but only of small sections; The big picture has been lost.
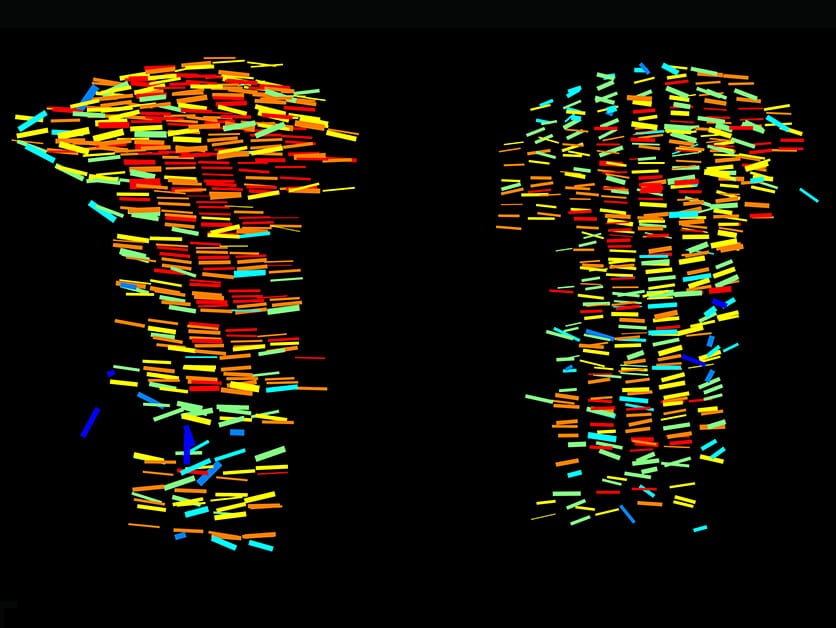
In the new study, led by Sarah Rubin, a research student in Prof. Seltzer's laboratory, the scientists developed the 3D MAPs method, which allows examining hundreds of thousands of cartilage cells in XNUMXD. The method consists of several steps: first, making the cartilage tissue transparent using chemicals and examining it under a light sheet microscope; Next, the obtained microscopy images are divided into segments, and the cells in each section are characterized using a series of algorithms and artificial intelligence tools; In the third and final step, we return to the full image and map the cells on top of it until a detailed XNUMXD map of the entire growth plate is obtained, which includes information on the shape and size of the cells, their position in space and their directionality.
Thanks to the unprecedented detail of the method, the scientists were able to disprove a common assumption about bone growth. It is known that the cells of the growth plate are arranged similar to archaeological layers: new cells are born at the ends of the bone and older cells that have undergone various growth processes are located closer to the center of the bone. In previous studies, it seemed as if the "oldest" cells, meaning those closest to the center of the bone, were almost ten times larger than the cells adjacent to them. However, when the institute's scientists examined mouse growth plates using the new method, they discovered that the supposed jump in cell size is actually an illusion resulting from the XNUMXD imaging. In reality cells grow much more gradually.
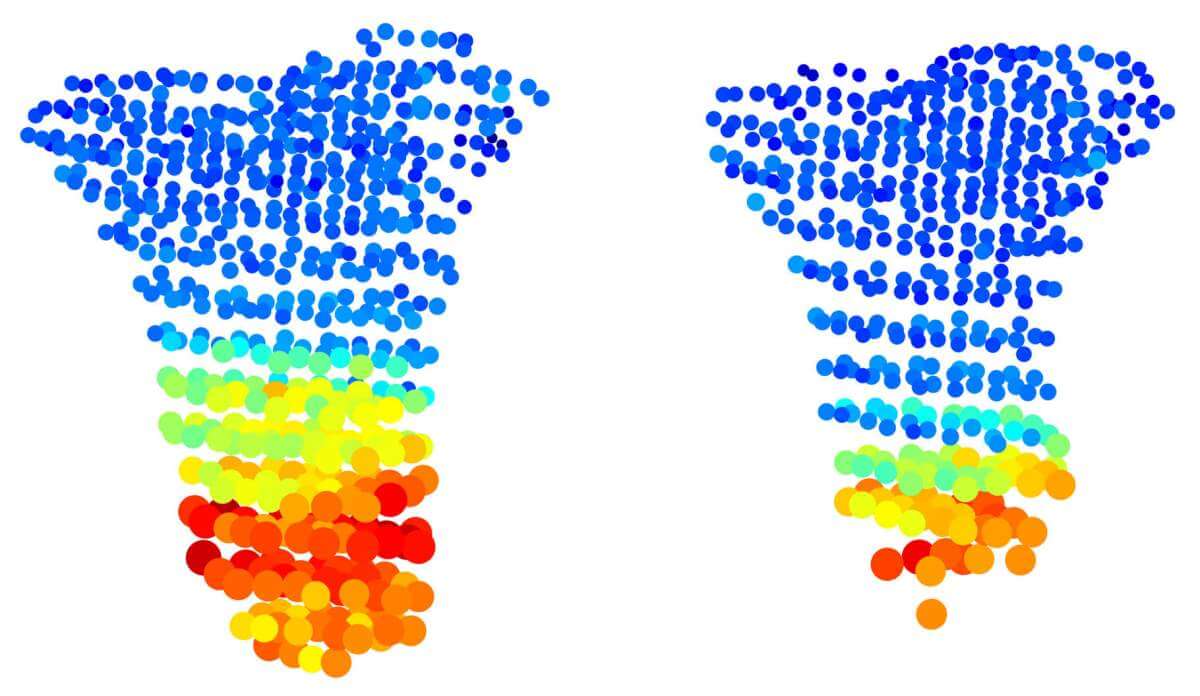
The method also allowed the researchers to decipher two mysteries concerning a type of dwarfism called Grebe syndrome. It is known that this syndrome results from a mutation in the gene Gdf5, but it was not clear how a mutation in the gene plays a role in the formation of joints, leading to short and deformed limbs. Moreover, the growth plates of the loci in the syndrome appear to be completely normal. When the scientists tested transgenic mice with a gene mutation Gdf5 Using the new method, they found a series of disruptions in the growth plates that had not been discovered in previous studies. In doing so, the researchers revealed that apart from creating joints, the gene also plays a role in regulating growth processes, and that a mutation in the gene leads to the fact that the cartilage cells do not grow at the right rate, their shape is not normal and they are not oriented in the right direction. "Imagine marbles packed in a bag - if we change their shape, we will no longer be able to arrange them in the bag in the same way," says Robin. "Thus, even the deformed cells in the growth plate are not organized correctly, and this prevents the bone from elongating properly."
The new method will allow scientists to continue to study the processes that cells go through in the growth plate - both in normal growth situations and in various growth disorders. This method will also make it possible to study external influences on bone growth, for example, the influence of the muscular system. Prof. Seltzer concludes: "If the growth plate is the 'engine' that drives bone growth, we lifted the hood to see closely how it works and what happens when it breaks down."
Dr. Ankit Aggarwal, Sharon Kriaf, Dr. Neta Plezenthal, Yonatan Saburai and Dr. Tomer Stern from the Department of Molecular Genetics also participated in the study; Dr. Yosef Addi from the Department of Life Sciences Research Infrastructures; Prof. Johannes Stegmeier from RWTH Aachen University; and Prof. Paul Viotra from the University of Aix-Marseille.
More of the topic in Hayadan:
- The strange and "noisy" method in which leaves are used to grow
- Eating "junk food" during the growth period damages the normal development of the bones, even with moderate consumption
- The concept of "green growth" will not save the planet, but decision makers fear a world without growth
- Tel Beit Yarah: New Evidence of Connections with Egypt's First Dynasty
- The 'immortal strand' hypothesis is disproved
