The treatment met the criteria of efficiency and safety; Phase 2B of the clinical trials is expected to begin later this year
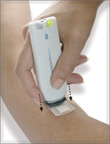
Transpharma Medical, a biopharmaceutical company specializing in the development and marketing of medical products based on a unique technology of drug delivery through the skin, announced today the successful completion of Phase 2A of the human clinical trials of ViaDerm-hPTH(1-34) for the treatment of osteoporosis Closed).
In June 2008, the pharmaceutical giant Eli Lilly and Transpharma signed a licensing and development agreement for ViaDerm-hPTH(1-34), a medical product developed by Transpharma based on the PTH (1-34) peptide that encourages bone building. As part of the development, Transpharma completed a three-month phase 2A clinical trial for transdermal PTH(1-34). The treatment met the primary and secondary efficacy and safety goals defined in advance, including the safety of the skin after repeated use of the ViaDerm system. More details on the results of Phase 2A of the study are expected to be published during 2010 at one of the leading international conferences on osteoporosis. Phase 2B of the trials will also begin later this year, which will test dosage ranges. This study will be conducted jointly by Eli Lilly and Transpharma.
"We are very satisfied with the results of the trial, which indicate impressive progress in the use of the ViaDerm system in clinical trials," said Dr. Dafna Hefetz, CEO of Transpharma Medical. "We met the primary and secondary objectives of phase 2A of the clinical trials, we are preparing for phase 2B of the trials and hope that in the future we can offer improved treatment to people suffering from osteoporosis by providing an alternative to daily injections of PTH."
About the ViaDerm system
The ViaDerm system for delivering drugs through the skin includes a palm-sized device that operates using batteries and creates microscopic passages in the outer layer of the skin that allow the passage of a variety of drugs that are on patches. The system provides a solution for self-administration of drugs in a cost-effective and simple way to operate and enables safe and accurate transfer through the skin of a wide variety of drugs, including water-soluble small molecules, peptides and proteins.
About osteoporosis
Osteoporosis (bone depletion) is a serious disease that affects approximately 75 million people in the US, Europe and Japan. Osteoporosis, which means "porous bone", is a disease that impairs bone density and quality. The bones are thinning, becoming more brittle, and the risk of fracture increases significantly. Bone loss is a continuous and symptomless process until the moment the first fracture occurs. The most common fractures due to osteoporosis are in the femoral neck, spine and wrist. The frequency of these fractures, mainly in the femoral neck and spine, increases with age in both women and men. Vertebral fractures may have serious consequences, such as loss of height, severe back pain and deformities.
