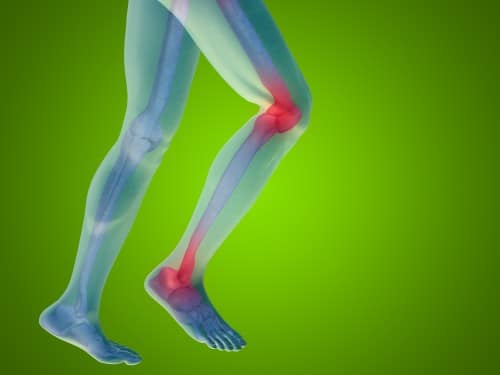
Chronic pain in all its forms - burning, prolonged pain, stabbing - may defeat any attempt to treat it. New insights into the causes of pain bring new ideas to combat it.
"Stop at the grocery store, not Burger King," Gemma Bond instructed her husband on her cell phone while rushing to buy ice cubes one night in 2012. "Their ice cubes melt too fast." Bond, 38 at the time and nine months pregnant, needed large ice packs to keep the water in the tub where she dipped her swollen, red and painful feet as cold as possible. She learned to cover her feet with garbage bags so the ice wouldn't hurt her skin. A few months earlier, Bond was a young, healthy woman who worked in the office of a company that installs solar panels, and lived a fairly normal life. Now she can hardly break away from the relief provided by the cold bath water, except for taking a shower, "which was like real torture."
Bond, who lives in Santa Rosa, California, suffered from a disease called erythromelalgia, or EM for short, a term that means "red limb pain" in Greek. The disease causes a burning and excruciating pain in the palms of the hands or feet and they become extremely sensitive to even lukewarm temperatures and to the slightest pressure. For most of those who suffer from the problem, it appears without any explanation (no connection to pregnancy is known). Although EM is a rare disease, affecting approximately 13 out of a million people, chronic pain in its many forms is surprisingly common and its origins are sometimes extremely obscure.
The number of Americans who suffer from chronic pain is estimated at about 100 million, and it usually appears in the form of back pain, headaches or joint pain. All in all, in the US, chronic pain affects more people than diabetes, cancer and heart disease combined, and it also costs more: according to a data analysis done in 2012, the cost of medical treatments and lost work days is about 635 billion dollars. And the price of suffering is incalculable: people facing severe hardship are at high risk of disability, depression, sleep disorders, drug and alcohol addiction, and suicide. Linda Porter, consultant in the field of pain management policy at the National Institute of Neurological Disorders and Stroke, and head of the Office of Pain Management Policy at the US National Institutes of Health (NIH), testifies that chronic pain is "a huge public health problem that is under-recognized and under-treated."
Pain exists for a reason. It acts as an internal warning system that warns of harm to the body, forcing us to quickly withdraw our hand from hot hands before it is badly burned, or to stop stepping on a broken leg. But sometimes the pain persists long after the threat has passed. Although chronic pains can appear without any explanation, in general they can be divided into two categories: inflammatory pains, such as pains caused by rheumatoid arthritis, for example; and neuropathic pain that usually results from nerve damage due to injury, disease or some other damage.
Chronic pain is notoriously difficult to treat, and neuropathic pain is particularly difficult, partly because anti-inflammatory drugs such as ibuprofen (a relative of Optalgin) or naproxen have almost no effect on them. Morphine and other opiates are the definitive answer to severe short-term pain. But their use involves side effects, starting with mild problems such as constipation and drowsiness, and ending with potentially fatal respiratory depression. People who use these drugs over time develop a tolerance and need ever-increasing doses of them, increasing the risks. Addiction and abuse are serious side effects associated with the use of opiates. More Americans die from prescription painkiller overdoses than from cocaine and heroin overdoses combined. Other medications currently used to treat chronic pain include agents originally used to treat convulsions and depression, and they also have limitations. Despite the expected risk to her unborn baby, Bond was given a cocktail of opiates, anticonvulsants and antidepressants to help her sleep and ease her sense of desperate distress.
Despite all the many efforts, science cannot offer safer and more effective drugs, but the situation is starting to change. Recent discoveries have opened new channels for drug development. "Researchers are now making impressive progress by focusing on the molecular pathways that signal pain," says Porter. "There is reason for hope."
Relay race
To understand these new attempts to treat chronic pain, it is advisable to know how pain is created. Pain begins as a stimulus picked up by nerve cells that are sensitive to pain and are called nociceptors, whose sensors are spread over the surface of the body in the layers of the skin. Stimuli that endanger the body, such as extreme high or low temperatures, strong mechanical force or dangerous chemicals, cause the excitation of these nerve endings. The nerve endings send signals to the cell bodies of the nociceptors found in concentrations of nerve cells called ganglia. The ganglions are located in the nerve roots coming out of the spinal cord, right at the points of their exit from the spine. The nociceptors transmit the threat message to nerve cells located in the spinal cord. These in turn stimulate the branched neural network associated with pain generation in the brain, which also includes areas involved in thinking and feeling (a fact that explains why placebos and distraction can sometimes relieve pain).
Like all nerve signals, pain signals travel quickly from one end of the nerve to the other using an electrical impulse called an action potential. The action potential is created due to the flow of ions, charged atoms of sodium and potassium, through the nerve cell membrane. The ions pass through tiny holes in the membrane called ion channels. These channels are actually protein molecules embedded in the nerve cell membrane and change their shape from an open state to a closed state under the influence of the ion concentrations in their environment. Special ion channels at the ends of nociceptors detect various threats such as extreme heat or substances leaking from nearby damaged cells. When the channels open, positive ions flow into the cell and cause a slight change in the electrical voltage on both sides of the membrane. This voltage change in turn activates additional ion channels that are highly sensitive to voltage changes. When such ion channels open in numbers that exceed a certain threshold, the resulting flow of ions triggers an action potential that races rapidly along the nerve cell. The action potential reaches its peak when it causes the release of a neurotransmitter in the spinal cord, a chemical messenger that transmits information to a nearby nerve cell.
Most of the information learned about pain in the last twenty years centers on ion channels: how they detect signals such as heat or tissue damage; which of them are needed to create a pain signal, compared to others that play secondary roles; And the most pressing question, what are the channels that must be acted upon to silence pain signals safely.
Researchers and pharmaceutical companies realized a long time ago that blocking sodium channels in nerve endings relieves pain; The short-term anesthetics lidocaine and novocaine, for example, block sodium channels not only to dull pain, but all sensations in the places where they are injected. Nine voltage-gated sodium channels have been identified in humans and other mammals, each of which opens in response to slightly different voltage differences. Blocking all of them will cause a devastating result, since sodium channels are found in all nerve cells in the body, including in the brain and heart. A blanket blockage of all of them may interfere with the passage of signals responsible for the heartbeat, breathing and movement. That's why scientists have been looking for a redeeming solution for years: sodium channels found only in pain-sensitive cells.
In the late 90s, researchers came close to achieving this goal following the discovery of three voltage-gated calcium channels found only in the peripheral nervous system (ie, neither the brain nor the spinal cord), where pain signals normally originate. All three, named NaV1.7, NaV1.8 and 1.9NaV, are mostly found in nociceptors and some other nerve cells involved in creating sensations. (The letters Na indicate sodium, V indicates voltage, while the number indicates the position of the channels in the family of the nine known channels.) Once the genes encoding these channel proteins were identified, researchers were able to manipulate their action in laboratory animals. Experiments conducted over the next ten years confirmed that, at least in mice, silencing sensory NaV reduced neuropathic pain.
Since 2000, developing drugs to target NaV channels has seemed a promising target, but drug companies needed more evidence than animal studies to justify significant investment in development. The necessary data came from four prominent papers that linked NaV1.7 to pain in humans. In 2004, a research group working in Beijing found mutations in the gene encoding NaV1.7 in two Chinese families who suffered from hereditary erythromelalgia, the same disorder that appeared in Gemma Bond spontaneously during her pregnancy. In 2005, Steven Waxman and Suleiman Dib-Haj, working at Yale University School of Medicine and the Connecticut Veterans Health System, confirmed that these mutations led to an overactivity of NaV1.7 that was there to cause pain. Shortly thereafter, John Wood and his colleagues at University College London reported that another disorder, brief spasms of extreme pain causing severe pain in the rectum, eyes and jaw, was due to overactivity of the NaV1.7 channel due to a mutation. Particularly decisive information was obtained from Jeff Woods and James Cox who were then working at the University of Cambridge, according to which mutations in NaV1.7 that completely paralyzed its function, simultaneously eliminated all sensation of pain, and caused a rare and dangerous disorder that often leads to death from injuries that the person does not feel including a rule. Combining all the findings regarding abnormal genetic conditions confirmed the importance of the NaV1.7 channel as a cause of pain sensations in humans.
Waxman studies rare genetic diseases because, according to him, despite their rarity, they may be useful as "markers that teach about pathological pathways that may be more common." In 2012, in collaboration with researchers from the Netherlands, he made the leap from the rare to the more common. The term "small fiber polyneuropathy" is a broad label used to describe damage to pain-sensitive nerves in the peripheral system, usually in the hands or feet. In about half of the patients diagnosed with this disorder, an identifiable source of nerve damage is found, such as diabetes, but in the other half the cause of the pain remains unknown. Waxman and his Dutch partners tested the DNA of patients with unexplained pain and found mutations in the NaV1.7 genes in almost 30% of them. In 9%, mutations were found in NaV1.8 genes, and in another 3%, mutations in NaV1.9. Waxman's group also found that people suffering from chronic pain after nerve injury have a larger than normal number of NaV1.7 channels in the damaged nerves.
These findings were enough for the pharmaceutical companies to start investing in serious work in the search for sodium channels unique to sensations. For several years, Pfizer has been developing drugs targeting NaV1.7 and NaV1.8. Neil Castle of the Neusentis Group, Pfizer's pain and sensory disorders research unit in Durham, North Carolina, says it's too early to say when the new painkillers will hit the market, but several are currently being tested in patients. Unlike drugs in use such as lidocaine, these new molecules do not target the main opening of the calcium channel, which is almost the same between the different groups of calcium channels. Instead, they act on an area of the channel that senses the electrical voltage and it is different from one channel to another, which gives them higher specificity and probably makes them safer to use. In 2013, Castle's group reported the discovery of a chemical that selectively damages the NaV1.7 voltage sensor. Such molecules, says Cassel, "are extremely selective, so they do not affect the heart or muscle function," at least not in the early tests.
Meanwhile, a team at Duke University is also targeting NaV1.7's stress sensor, but is doing so using an antibody, a molecule of the immune system. According to a study published in June 2014, the antibody relieves inflammatory pain and neuropathic pain in mice, and it also moderates itching, thus giving the drug the ability to be "three-for-one" in the realm of pain medications. Researchers testing the ability of certain components in animal venom to act on NaV1.7 are also having some success (see below).
They get hot
Calcium channels are not the only targets of researchers' efforts. Another ion channel found almost exclusively in pain-sensing cells is called TRPV1 and is known to be activated at high temperatures and in response to capsaicin, the substance that gives chili peppers their spiciness. Since David Julius and his colleagues at the University of California, San Francisco discovered the gene encoding TRPV1 in 1997, many scientists have been eagerly searching for molecules that would silence pain signals by blocking this channel.
"TRPV1 has been such a promising, yet so elusive, target for a long time," says NIH's Porter. Early blocking agents that closed it had uncontrollable side effects, such as overheating of the body and loss of sensation to heat that could cause burns. The canal, which also senses acid, spider toxins and inflammatory substances, was recently discovered to be a complex factor that combines several sensory signals together. The best medicine will not undermine the core function of the canal, the ability to feel heat," says Julius. It will only calm an overactive channel.
Julius' team took another step in December 2013, when they published the first high-resolution images of the structure of TRPV1 in different states. This information may help researchers discover how to block the channel only when it changes to the shape that triggers the pain.
Misinterpretation of pain
Most people suffering from neuropathic pain experience its three most prominent representatives: hypersensitivity to painful stimuli; Spontaneous pain that attacks unexpectedly and allodynia, a condition in which completely normal touch causes pain. (Because of allodynia, dripping shower water was torture for Gemma Bond.) The study of ion channels helped explain the hypersensitivity, and research in another direction showed how allodynia develops. In the normal state, signals of pain and signals of non-painful touch travel in separate pathways from the cells in the skin to the spinal cord and from there to the brain, but in allodynia, the signals cross in the spinal cord: touch-sensitive nerve cells excite the pathway of pain sensations.
The question of what is going wrong there was investigated mainly by researchers in Japan and two groups in Canada, one led by Yves de Koninck from the University Institute of Mental Health in Quebec, and one led by Michael Salter from the Children's Hospital in Toronto. In animal studies, they found that in response to nerve injury, microglia cells that protect the nerves, release a signal that causes nerve cells in the spinal cord to reduce the number of protein molecules, ion carriers, called KCC2 (KC is short for potassium chloride). The carrier maintains the delicate balance of chloride ions inside and outside the cells. Under normal conditions, small nerve cells in the spinal cord called intermediate cells regulate the communication between the pathways of pain sensations and non-painful sensations. They do not allow normal contact to cause pain and allow a soothing caress to relieve the pain temporarily. But when nerve cells in the spinal cord lose KCC2, that communication goes awry, and light touch can trigger pain. The researchers hypothesized that restoring KCC2 to its normal level would stop the signaling disruption.
In November 2013, de Koninck and his colleagues reported the discovery of a compound that increases chloride transfer through KCC2. The drug restored the balance of chloride ions and the electrical function in the nerve cells in the spinal cord. Moreover, it induced neuropathic pain relief in rats. The KCC2 enhancer was safe to use and had no side effects in animals, even when given at high doses.
Although the work has so far only been done in animals, certain aspects of the KCC2 carrier indicate that it is an excellent target for the development of human therapies. Unlike other drugs that inhibit the action of ion channels comprehensively, the factor that increases the passage of chloride should affect, for example, only damaged cells, says de Koninck. Cells that have normal and functioning KCC2 will continue to function normally and the drug will not increase their activity excessively. The experiments show that the drug does not necessarily affect the way KCC2 functions, but leads more carrier molecules to the cell surface area. A fuller understanding of this movement control is essential to the development of safer and more effective pain relievers.
Personalized pain management
Most researchers believe that the future of medicine is in personalized medicine: the genes and special sensitivities of each person will dictate the best course of treatment and the safest way to prevent disease. In the field of chronic pain management, this future is just beginning to loom on the horizon. "It would be great if we could say exactly what went wrong with each patient. In such a situation we can say, 'Hey, you're going to get this drug, and you're going to get that drug,'" says David Bennett, a neuroscientist at the University of Oxford. But treatment, provided even in the best comprehensive pain treatment centers, relies mostly on trial and error.
Now patients carrying rare mutations in NaV channel genes are helping to pave the way for personalized pain treatment. For example, most people who suffer from the burning limb pain of erythromelalgia due to an inherited mutation in NaV1.7 cannot benefit from carbamazepine, an anticonvulsant drug that is sometimes used to relieve pain. However, one family suffering from the disease has a unique mutation (there are many types) that responds well to this drug. By investigating the molecular structure and function of the mutant channel in this family, Waxman and Div-Haj were able to show how carbamazepine moderates the overactivity of the channel, and subsequently were able to accurately predict that it would also be effective in a slightly different mutation. These findings are exciting, Waxman says, because they suggest that basing treatment on a person's genetic make-up is "not impractical" for patients with hereditary erythromelalgia as well as those with more common pain problems.
As for Gemma Bond, her symptoms suddenly stopped right before she gave birth to her son, a few weeks premature. Unexpectedly, steroid injections designed to help mature the newborn's lungs worked like magic on the mother. "I woke up in the middle of the night," she recalls, "and my feet didn't hurt, which hadn't happened in over six months." No one could explain why. The symptoms have indeed returned since then, but in no case with the intensity that caused her suffering during the pregnancy. "If I stand on my feet for a long time, it has a direct consequence: they will hurt," says Bond. "I'm coping with it, and now I'm living without medication, so it's really wonderful. But I will be happy if they manage to cure me." And the pain researchers would love to bring relief to Bond and millions like her.
in brief
Chronic pain affects more people and costs more than cancer, heart disease and diabetes combined.
Opiates and other drugs in use fail to relieve chronic pain, and carry serious risks.
Discovery of unique molecular pathways for pain has revealed new targets for drug development. Substances found in animal venom are being tested as next-generation pain relievers.
on the notebook
Stephanie Sutherland is a neuroscientist and science reporter working in Southern California.
A chance for treatment
Hints for pain relief
Pain signals generated due to heat or other stimuli travel from nerve endings in the skin or elsewhere to nerve structures in the back called root ganglia ("root" is a term for the place where a large nerve exits from the spinal cord), located near the spinal cord, and from there they travel to the spinal cord and the brain. Gene mutations or nerve cell damage can change the activity of key molecules along this pathway, including ion channels, in a way that causes pain to become chronic. Researchers hoping to alleviate suffering are now focusing on these key molecules in a variety of ways.
Hyperactive channels
Within the membranes of the nerve endings that detect pain stimuli, protein molecules are embedded which are actually ion channels, which open and close a pore located in their center in response to stimuli. A channel called TRPV1, for example, detects a heat stimulus. When it opens, positively charged ions (mainly sodium) flow in and raise the membrane tension. In response, voltage-sensitive sodium (NaV) channels open and trigger a pain signal that reaches the spinal cord. Structural disruptions in NaV or TRPV1 can lead to multiple signaling. The materials currently being researched may reduce the activity of channels and thus curb the excess of signaling.
Cables get tangled
Some nerves that detect sensory input specialize in transmitting pain sensations; Others convey a sense of touch. The communication between these two pathways is regulated by cells in the spinal cord called intermediate cells (in blue). In people who suffer from chronic pain, this regulation sometimes goes wrong, so they suffer from allodynia, pain that originates from a harmless stimulus such as gentle touch. The research shows that this disorder can arise after a nerve injury, when microglial cells release chemical signals that cause nerve cells in the spinal cord to lose a molecule essential for normal signaling. The drug developers are working on ways to correct such shorts and relieve the pain of allodynia.
why me?
A variety of factors can explain why some people are more vulnerable than others to chronic pain
Take ten people who suffer the same back injury due to a car accident: three of them may be unlucky enough to suffer chronic pain after the accident. Or look at ten people with diabetes: about half of them will suffer from nerve damage or neuropathy, but the damage will cause lasting pain in only three of them. What are the factors that make some people vulnerable, compared to others who are strong? There is still no complete answer to this question, but studies point to three main influencing factors, which apparently work together:
One is built-in: genes play a role in determining each person's sensitivity and tolerance to pain, and some of them tip the scales toward abnormal hypersensitivity to chronic pain. One of the most prominent genetic factors is genetic gender; Women's chances of suffering from chronic pain during their lifetime are much greater than men's.
Early experience: stress, trauma and abuse, physical or emotional, may increase the risk. Studies show that such experiences can bring about long-term changes in gene activity, activating or silencing genes in ways that affect pain sensation pathways. Also, the risk of chronic pain increases with age, not only because of the general wear and tear but probably also because of the decrease in the body's ability to repair injuries, including nerve damage.
Personality: Certain personality traits bias the level of risk. Pessimists, particularly anxious people, and those who tend to expect disasters, are more likely to suffer from chronic pain than others. The neural connections in the brain involved in the creation of motivation and the feeling of reward also probably affect the vulnerability to pain.
More ideas for treatment / Mark Peplo
Take the sting out of the pain
Venom molecules may provide alternatives to addictive drugs from the opiate group
Glenn King milks the venom of swindlers, and it's no easy task at all. "We tie them up with elastic bands, put electric pliers close to their pincers, apply voltage, and they emit venom," says King, a biochemist at the University of Queensland in Australia.
It is possible that the liquid with the minimal volume contains substances that could serve as the basis for a new series of pain relievers. Venom fluids of various types are natural reservoirs of nerve-paralyzing molecules, and with 400 different types of venom in his lab, King is at the forefront of the effort to identify pain relievers in the venom of nettles, spiders, snails and other venomous creatures.
Major pharmaceutical companies are scrambling to synthesize alternatives to addictive pain relievers such as morphine, but they are having trouble creating molecules that target only the specific nerves that need to be paralyzed. Animal venom, on the other hand, has evolved naturally and contains molecules endowed with this particular specificity. In laboratory animals, these molecules paralyze nerves without harming the rest of the body. The molecules that many researchers are trying to reach are called voltage-gated sodium ion channels, and they are common in pain-sensitive nerve cells. Blocking a certain channel, known as NaV1.7, prevents the cell from transmitting a message of pain to other parts of the body, as described in the article here.
Certain components of venom have a structure and chemical activity that are just right for sticking to a part of the channel called a voltage sensor, and when they stick to it they block the channel. In 2013, King identified a venom molecule known as m-SLPTX-Ssm6a, which is believed to be the most selective NaV1.7 inhibitor ever found. King found it in the venom of the red-headed Chinese handel (Scolopendra subspinipes mutilans), which can reach 20 centimeters in length and is equipped with a pair of brutal-looking pincers. "If they catch you, it hurts a lot," says King. However, the molecule had the opposite effect on injured mice: in experiments it relieved pain more effectively than morphine. And it had no unwanted effects on blood pressure, heart rate or motor functions, a fact indicating that it does not affect the central nervous system, as an opiate such as morphine does.
King's team produced a synthetic version to test whether a drug could be made. But to the dismay of the researchers, this version did not work with the same degree of success. King suspects that the original formulation of m-SLPTX-Ssm6a contained traces of another active ingredient. He is working on another round of milking Nadalim to search for the mystery ingredient.
Snake venom is also a source of selective channel blockers. Anne Baron, a pharmacologist at the Institute of Molecular and Cellular Pharmacology in France, isolated two pain-relieving molecules from the venom of a black mamba. "We are almost ready for a clinical trial," says Baron. "We did a lot of experiments on rodents to gauge the toxicity." Mambalgines, as the molecules are called, block a certain group of acid-sensitive ion channels in peripheral nerve cells, which, like the sodium channels, allow the cells to transmit pain signals. Coincidentally, membalgins have no effect on most other ion channels, which may explain why mice given injections of these substances suffered no apparent side effects.
Precise damage to nerve cells is not the only target of venom research, says David Craik, a biochemist in Queensland. If venom molecules are to be found in painkiller pills, they must be resistant to breakdown in the digestive system. In 2004, the US Food and Drug Administration approved for use a pain reliever called ziconotide, based on a molecule isolated from the venom of the Conus victoriae snail. But the drug did not survive the harsh conditions prevailing in the stomach, so it must be injected into patients with a slow injection pump, a cumbersome and burdensome process. "Ziconotide is not selling well," says Craik.
Crake began to re-engineer pain relievers derived from the snail's toxins. His strategy is to try to make the molecules, which are chains of amino acids, into rings. Rings are much more stable structures and the enzymes in the body cannot cut their ends. The compound, dubbed cVc1.1, was found to be 100 times more potent than gabapentin, a common pain reliever. This year and in early 2014, at the meeting of the American Chemical Society in Dallas, Texas, he revealed five ring conotoxins that showed resistance in early experiments. Since there are tens of thousands of poisonous species in the world, the researchers believe that it is only a matter of time until the compound is found that will hit the desired target, will be immune to decomposition and will be able to be produced in large quantities without difficulty. "We know maybe one percent of the components found in the venom of these animals," says Baron.
About the author
Mark Peplow is a science reporter working in London.
for further reading
Black Mamba Venom Peptides Target Acid-Sensing Ion Channels to Abolish Pain. Sylvie Diochot et al. in Nature, Vol. 490, pages 552-555; October 25, 2012.
Discovery of a Selective NaV1.7 Inhibitor from Centipede Venom with Analgesic Efficacy Exceeding Morphine in Rodent Pain Models. Shilong Yang in Proceedings of the National Academy of Sciences USA, Vol. 110, no. 43, pages 17,534-17,539; October 22, 2013.
Pain Vulnerability: A Neurobiological Perspective. Franziska Denk, Stephen B. McMahon and Irene Tracey in Nature Neuroscience, Vol. 17, pages 192-200; February 2014.
Regulating Excitability of Peripheral Afferents: Emerging Ion Channel Targets. Stephen G. Waxman and Gerald W. Zamponi in Nature Neuroscience, Vol. 17, pages 153-163; February 2014.
Can this pain stop? Alan A. Basbaum and David Julius, Scientific American Israel, October-November 2006.
Pain toxin, Gary Stix, Scientific American Israel, August-September 2005
The article was published with the permission of Scientific American Israel

16 תגובות
I heard that cannabis treatment can help a disease called "erythromelalgia"... why don't you write about it???
Sometimes the source of the pain is in another area of the body and radiates to other areas.
Manual treatment by a qualified person and professional diagnosis can relieve the pain in a relatively short time
In the Hebrew language there is a term called "nerve fountain".
This term is used a lot in borrowing, to describe a center of activity.
Once, I tried to find out if there is a "map" that describes the nerve centers
which come out of the human body.
No one could show me such a map...
So are there or are there nerve centers in the human body?
In the Hebrew language there is a term called "nerve fountain".
This term is used a lot in borrowing, to describe a center of activity.
Once, I tried to find out if there is a "map" that describes the nerve centers
which come out of the human body.
No one could show me such a map...
So are there or are there nerve centers in the human body?
Without much ado, CAPSACIN - God's gift against pain.
Great article. It simply illustrates in a great way the profound ignorance that a large part of humanity has about the human body.
Moreover, it is extremely difficult for me to understand how those researchers do not see the mental distortion leading their research.
I apologize for sounding prickly and critical in opening my words. Nevertheless, I would ask that you read them with openness and from a healthy scientific and research approach.
The mental distortion can be illustrated in a simple way. Suppose there is an alarm system that alerts every time missiles are fired at Israel. At a certain point, the residents of Tel Aviv start to suffer from the loud alarm and see it as the source of the problem, and start looking for ways to sabotage it in order to "not suffer".
This is exactly what the researchers are doing in the described article, by looking for ways to paralyze the body's nervous system.
In this article there is one paragraph that begins with the words: "There is a reason for the existence of pain", and the explanation corresponds to what I described about the alarm system. The pause then changes to: “But sometimes the pain persists long after the threat has passed. Although chronic pain can appear without any explanation."
At this point, the researchers' approach fails in that it assumes that the pain continues after the problem (the missiles) has been resolved, or that unexplained pain appears. They are right that they don't have an explanation for the continued appearance of the pain, but that doesn't mean it doesn't have a reason. It's not because the alarm system went down.
As someone who is engaged in research on the nervous system, I will share with you several discoveries.
First, chronic pains, that is, persistent pains - seemingly for no reason, exist in all people, in almost all parts of the body.
These pains are the result of a variety of factors (illness, overexertion, wrong diet, smoking, stress, anxiety, anger, exposure to radiation, etc.).
Anything that harms the body causes pain of a certain intensity.
For a reason that is not clear to me yet, at some point we stop feeling this pain, even though the problem is still there.
In the meantime, the problem gets worse until at a certain point we can no longer feel the pain, and then we call it chronic pain.
One of the discoveries of the research showed that if a person focuses his attention on any organ, whether it is a toe, heart, teeth, and so on, for a long enough time, he will feel the pain again.
In fact, this is a simple experiment that anyone can do.
Choose a certain organ, for example the foot, and focus your attention regularly on the sensations in the foot.
At first you may not feel anything, but if you persist in focusing your attention on the foot, let's say for 20 minutes, at some point you will feel that it is full of pain.
As you continue the pain will only intensify. It is not because the process creates damage to the leg, the opposite is true, but the focus of attention, in a way allows the pain that is there to be experienced.
Whoever tries to do this exercise on the different parts of his body will find that his body stops a huge lump of dormant pain.
The most interesting point is that if you persevere long enough (it can take hours), focusing your attention on the feeling of pain, at a certain point it begins to diminish and a tremendous relief is created in the body, and a feeling of lightness that was not there before.
In fact, a healing process was created there, which so far I have been able to apply to every chronic pain.
I still don't know how this happens, but this is the observation of the research, and practically anyone can experience it for themselves.
However, another important observation showed that if the cause of the pain is not removed, (for example anxiety), the pain will also return.
Another interesting finding is that areas that contain a lot of dormant pain correspond to areas where it is common to find various diseases in people, such as types of cancer, diseases of the digestive system, heart attacks and the like.
In the bottom line, in relation to the above article and the approach described in it, I want to emphasize that pain is not a problem or a disease that must be solved, pain is an alarm system for problems that exist in the body, even if we are not aware of their existence.
In my opinion, the development of stronger pain relievers, as a solution for treating chronic problems, will only worsen the condition of the sufferers, since, if thanks to the pain they could know that there was a problem and sought to treat it, the cessation of the chronic pain would only cause the problem to be felt, and a quick death.
And in conclusion, I will end with an interesting point for thought.
My neighbor died of a heart attack at the age of 60. Only three weeks before the attack he complained of chest pains.
A heart attack is the result of a cumulative process of years and not of three weeks.
In my opinion, any person curious enough to try to apply the exercise I described earlier to his heart, will find that his heart "screams" in pain.
However with my neighbor, he managed not to feel it until three weeks before he had the attack.
With all the canals, in the end, the last stop is the updating of the information in the brain
and turning it into a virtual feeling state
In the future it would be possible to connect directly to the brain, and change awareness and feelings as you wish.
Why so long!?
And once again, your class responds.
If you want to substantiate what Atav says then speak. Otherwise, don't talk.
Miracles
once again,
You don't deserve a response…
I didn't ask for a response, I didn't expect a response, and I'm surprised you responded.
I just wanted to balance your response with a slightly more grounded opinion. There are readers here who deserve more than your constant venom.
Miracles
As I have already written many times,
You don't deserve a response…
To my father Blizovsky,
Returns, Doz Poa on the scope and the depth.
Thanks for the epilogue with Papello soup.
Nisim
Is your comment worthy of reading?
Now you will log back in to the page to see what Safkany's reaction was so you can teach him a few bad words, but when you will see my comment you will attack me instead. Must be your father's site...
When someone who is not an expert in any field starts a comment with "there is no doubt that..." then there is no point in reading the rest of the comment.
An easy matter, for someone who is involved in the field and knows how much research is invested in the field.
There is no doubt that this is a failure of medicine in understanding the human nervous system.
The matter of chronic pain, which is not justified,
It is a serious matter of the first degree.
Apparently it ranks low in the allocation of public research resources.