Researchers from the University of Virginia in the US have revealed unique active sites found on a titanium dioxide substrate through spectroscopic and theoretical measurements.
Researchers from the University of Virginia in the US have revealed unique active sites found on a titanium dioxide substrate through spectroscopic and theoretical measurements.
Catalysts are used in a wide variety of fields, including the conversion of oil and renewable sources into fuel, as well as for the production of plastic products, fertilizers, paints, solvents, medicines and more. About twenty percent of household products in the US depend on catalysts to initiate the chemical reactions required to make the products.
Catalysts are substances that activate necessary chemical reactions without themselves changing in the process. This property allows catalysts to be used over and over again since they are not consumed or eroded during the chemical reactions in which they are involved. Chemists have long succeeded in discovering and developing many catalysts and continue to do so, although the exact details of their mechanisms of action are not always fully understood.
New research conducted at the University of Virginia describes for the first time ever a new type of catalytic site where a catalytic oxidation reaction occurs, thus shedding new light on the inner workings of the process. The research, conducted by John Yates, professor of chemistry, was published in the prestigious scientific journal Science.
The researcher notes that the new discovery has implications for the understanding of catalysis in a variety of materials, since oxidation catalysis is essential for a number of technological applications.
"We now have both experimental tools, such as spectrometers, and theoretical tools, such as computational chemistry, which allow us today to study catalysis at the atomic level," explains the researcher. "We are able to focus inside the mechanism and locate the exact point with an efficiency that is higher than ever before. The findings of this study could be useful for the development of catalysts for all types of catalytic reactions."
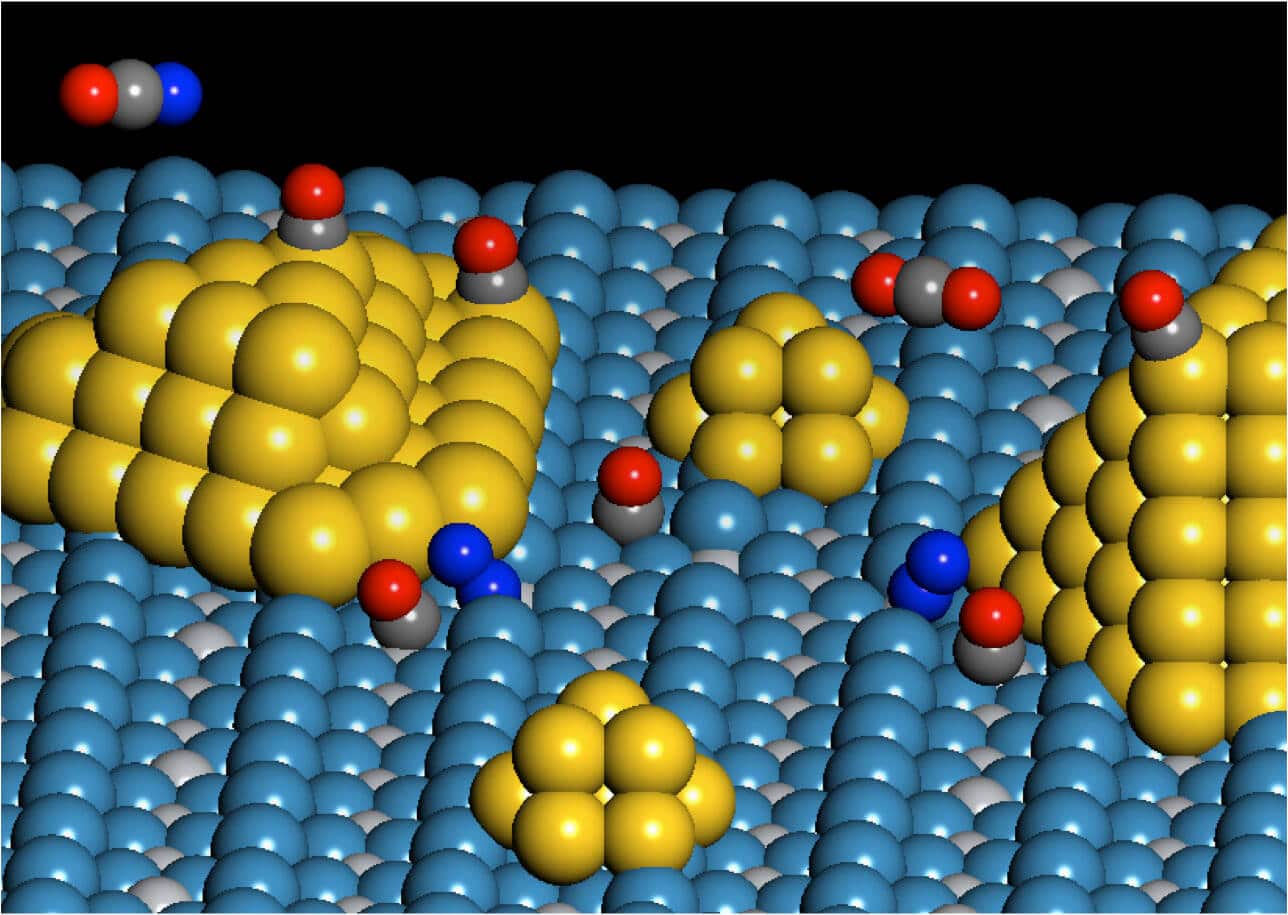
Using a substrate of titanium dioxide on which gold nanoparticles are placed, the researchers found a special site that serves as a catalyst in the peripheral area of the gold and titanium dioxide.
"The site is special because it is involved in the binding of an oxygen molecule to a gold atom and a nearby titanium atom located on top of the substrate," explains the researcher. "Neither the gold nor the titanium dioxide exhibit this catalytic activity when studied separately."
Using spectroscopic measurements combined with theory, the research team was able to track the specific molecular transformations and determine precisely where it occurs on the catalyst. The researchers proved that the significant catalytic activity occurs at specific sites that are formed in the peripheral area between the gold particles and their titanium substrate. "We call it a double catalytic site since two different atoms are involved in it," the researcher points out.
They observed that an oxygen molecule binds chemically both to the gold atom at the end of the gold cluster and to a nearby titanium atom in the titania substrate and reacts with a carbon monoxide molecule to obtain carbon dioxide. Using spectroscopy, the researchers were able to follow the consumption of carbon monoxide at the double site.
"This defined site is unique and is responsible for initiating the activation of the oxygen molecule undergoing an oxidation reaction on the catalyst substrate," explains the researcher. "This is a new family of previously undiscovered active sites."

One response
Very low understanding of humanity in chemistry. A bit surprising.
From the article it follows that there is currently no understanding of why the catalysis is created where it is created, in general. That is, there is something very basic in chemistry here that is not understood at all. There is no theoretical software tool that knows how to model atoms and molecules in general and the forces around them? and hence to conclude in advance where the activity sites will be...
We only know how to guess where there will be active sites based on imagination for familiar cases. And all they have managed to do now is to determine the location of another site. They did not understand at all why it was formed where it was formed or how it is involved in the acceleration of the reaction. I understand it right?