Scientists at the Weizmann Institute of Science have discovered that the venom of a sea snail called a poisonous cone works in a mechanism that was not known until now. The findings may help in the development of new drugs for hypertension and heart rhythm disorders

Inside the colorful shell of the sea snail, known as a poisonous cone, resides a deadly predator that quickly and efficiently destroys fish, worms and other passers-by that peck in its path. In a study that was recently published in the scientific journal Proceedings of the American Academy of Sciences (PNAS), Weizmann Institute of Science scientists revealed that the venom with which the venomous cone kills its victims works in a previously unknown mechanism. These findings may explain some of the side effects of drugs that block potassium channels, such as drugs for hypertension and heart rhythm disorders, and lead to the development of drugs that will work in new ways.
"Certain components in the venom of the venomous cone kill insects but not mammals," says Dr. Yizhar Karvat, a faculty scientist in the laboratory of Prof. Eitan Reuvani in the Department of Biomolecular Sciences. "We set out with the idea of using these ingredients to create natural insecticides that would not harm humans, but the research took a surprising turn."
Many venomous animals paralyze their victims with toxins that block the opening of the channels that carry potassium ions into and out of the cell. These potassium channels are essential cellular mechanisms for regulating the transmission of electrical signals in the body and many other processes. Some of the drugs for high blood pressure and heart rhythm disorders work in a similar mechanism to these toxins, called "cork in the bottle" due to blocking the opening of the channel. When Dr. Kerbett and his colleagues began to study venomous cones, they checked, first, if their venom also works by the "cork" method. But the experiments in the laboratory showed that it is not the same mechanism.
Certain components in the poison cone's venom kill insects but not mammals. We set out with the idea of using these ingredients to create natural insecticides that would not harm humans, but the research took a surprising turn"

To understand how the deadly snail's venom works, Dr. Kerbet and other team members, in collaboration with scientists from Tel Aviv University and Debrecen University in Hungary, created toxic crystals central to its venom, and deciphered the three-dimensional structure of these crystals. After that, they made an electrophysiological recording of the activity of the potassium channels and created genetic mutations to examine the way of binding and the mechanism of the effect of the toxins on the channel. The findings revealed that unlike the known potassium channel blockers, the toxins in the cone venom act on the perimeter of the channel and not on its opening. Later, the scientists conducted computer simulations using advanced versions of molecular dynamics, a technology that allowed them to examine the interactions between the venom and the channel proteins at the level of individual atoms. "One of the advantages of this technology is that it reveals the location not only of the components of the protein molecule, but also of individual water molecules and ions," explains Dr. Kerbet.
The simulations revealed that the interactions between the amino acids of the venom and those of the channel proteins disrupted the hydrogen bonds within the channel in a way that increased the flow of water in the intraprotein spaces around the channel opening. The increased current, in turn, caused the entire orifice to collapse, thus preventing it from passing the potassium ions through the cell wall. In other words, the channel stopped working, and the flow of potassium ions was stopped, but not by a "cork in the bottle" mechanism, but by a new mechanism acting on the perimeter of the channel.
This discovery sheds new light on the control of potassium channels in the living cell. It is known that these channels may collapse due to prolonged opening and increased ion flow - a process called "slow inactivation" - but the molecular mechanism behind this process was unknown. The findings of the study indicate that slow silencing is probably caused by increased water movement and structural changes that occur as a result in the scope of the canal opening.
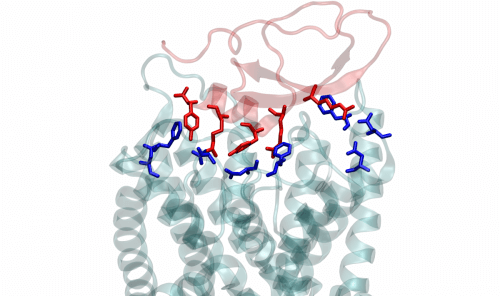
The findings also offer important insights for the development of future drugs. For example, it would be possible to check if some of the side effects of drugs that block potassium channels are caused by structural disruptions similar to those caused by the venom of the sea cone; And if indeed so, to develop drugs that do not cause these symptoms. The openings of the potassium channels have been preserved during evolution - that is, they have the same structure and the same molecular composition in different cells and cell types. This means that a drug that blocks the opening can affect all types of cells in the body. In contrast, the protein components of the canal perimeter differ greatly between different types of cells and tissues, so they can be much more targeted targets for drugs. "The development of drugs that will affect the scope of the channel and cause it to collapse, can be directed against a defined group of channels that are expressed only in certain cells," says Prof. Reuvani. "This may reduce the side effects of the drugs."
Shahar Payne and Dr. Dalia Gordon from the Department of Biomolecular Sciences of the Institute participated in the study; Shelly Hamer Rogotner and Dr. Orli Diem from the Institute's Department of Life Science Research Infrastructures; Hagit Altman-Guetta, Dr. Felix Frolov and Dr. Michael Gurvitz from Tel Aviv University; and Tivor Santo and Dr. Giorgi Penny from Debrecen University.

One response
How is the snail so smart?