The scientists of the Weizmann Institute of Science examined closely, and at high speeds, the activity of enzyme molecules, and showed that, unlike the machines we are familiar with, in these microscopic machines, randomness and noise are basic design principles
Random, noisy, and unpredictable - no engineer would put such a machine under his hands. It was also possible to assume that the microscopic "machines" in our bodies would operate by virtue of a similar logic. But a new study conducted in the laboratory of Prof. Gilad Haran At the Weizmann Institute of Science he examined closely, and at high speeds, the activity of enzyme molecules, and showed that in these molecular machines, randomness and noise are actually basic design principles. These findings, which may lead to a better understanding of the body's functioning at the molecular level, and even help in the design of new drugs or the development of efficient nanomachines, were published recently In the scientific journal "Records of the American Academy of Sciences" (PNAS).
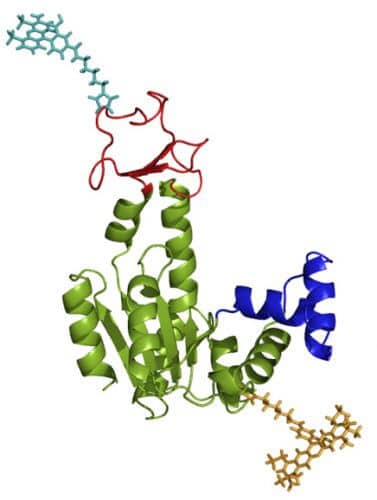
Prof. Hearn and his research group in the Department of Chemical and Biological Physics have developed precise methods for observing movements that occur in time sequences of microseconds (about a millionth of a second) in individual biological molecules, including enzymes. When an enzyme binds to a target protein, structural changes occur in it. In many enzymes, for a chemical reaction to occur, the two parts that make up the enzyme ("complexes" or "domains") need to meet. These configuration changes allow, among other things, to protect the active sites in the enzyme as long as they are not needed, and they help the enzyme locate the "substrate" - the target molecule to which it needs to bind. However, there is a debate on the question of how such a structural change, "closing the enzyme", is related to the biochemical phase of the enzymatic reaction.
In order to find out, the research team developed a method in which the enzyme molecules were isolated, and two tiny light-sensitive "antennas" were attached to them, one green and the other red, one for each of the complexes that meet when the enzyme changes its shape and closes. The green antenna was designed so that when a laser beam shone on it, it transferred its energy to the red antenna if they were close together, but it emitted the light back if there was a large distance between them. This is how the researchers obtained light emission in two colors - green in the open state, and red when the molecule is in its closed state. Detectors sensitive to the point of detecting single photons - with an accuracy of microseconds - picked up the light signals. "This is a leap forward in the resolution of time in experiments with single molecules," says Prof. Hearn.
The results revealed that the enzyme closed and opened feverishly and at a dizzying rate. Opening and closing cycles took place in less than 100 microseconds - a rate 100 times faster than the biochemical reaction rate. But why does an enzyme have to open and close 100 times instead of closing once and continuing to the next step? "We treat the activity of the enzyme as a mechanical function", explains Prof. Haran. "But enzymes are not characterized by any of the properties or conditions that we associate with machines. First, the enzyme is not rigid, but a flexible molecule that moves in a liquid. Second, and unlike a machine, where a piston moves inside a cylinder, and it is designed to perform identical repetitions of a certain action, a biological molecule is affected by 'noises' - various random changes in the environment. The 'noise' may be other molecules, fluctuations in temperature, or structural changes in the enzyme itself. And so, without an internal navigation system and under 'noise' conditions, the enzyme molecule needs to be able to perfectly direct the molecules to which it connects in order to prepare them for the chemical reaction."

Prof. Hearn and his colleagues concluded that the enzyme simply continues to open and close until, at some arbitrary point, it manages to direct the "substrate" in the most correct way for the reaction to occur. These findings indicate that randomness plays a more important role in enzyme dynamics than previously thought. Prof. Hearn explains: "In the 'noisy' environments where enzymes work, rapid random change may be the effective means even for performing the most precise and demanding operations. In other words, not only did these molecules adapt to operate under 'noise' conditions, they even adopted it as a method of operation."
After applying the ultra-fast method to one enzyme, Prof. Hearn and his research group are now investigating other biological molecules, with the aim of discovering rapid structural changes in them as well. "We hypothesize that processes on the order of microseconds are common in biological molecules, and we hope to reveal some of the rules and principles that underlie them," he says. "In 2016, the Nobel Prize in Chemistry was awarded for the 'design and construction of molecular machines' which may help in the development of new materials, sensors and means of energy storage. Today, these machines do not do much beyond simple movement on a track. To create better molecular machines, we may need to think less like mechanical engineers and more like enzymes, and embrace 'noise' and randomness as part of the design principles."
|
The enzyme molecule closes and opens feverishly and at a dizzying rate: opening and closing cycles took place in less than 100 microseconds - a rate 100 times faster than the biochemical reaction rate of the enzyme. #Science_Numbers
|

2 תגובות
Looks like a very interesting development platform. Me too in modesty. Save the link.
the article
http://www.pnas.org/content/115/13/3243