As part of dealing with these serious genetic diseases, the scientific community works on two levels: one is the development of technologies for early genetic diagnosis that will prevent the birth of sick newborns and the other is the research of the disease with the aim of curing it or at least alleviating some of its symptoms
By: Dalit Ben-Yosef and Ami Amit, Scientific American-Israel
Many genetic diseases are caused by a mutation in one of the genes. The mutation causes the creation of a damaged protein or lack of protein, resulting in disruption of the normal activity of a cell, tissue or system in the body. As part of dealing with these serious genetic diseases, the scientific community works on two levels: one is the development of technologies for early genetic diagnosis that will prevent the birth of sick newborns, and the other is the research of the disease with the aim of curing it or at least alleviating some of its symptoms. To study the various genetic diseases, transgenic laboratory animals are often used as models. In these animals, genetic engineering methods change the genetic makeup (genotype) of the animal so that it resembles the one that characterizes the disease in humans. The disadvantage of this method is that the manifestation of the disease in the transgenic animals is not necessarily the same as the disease in humans, which is partly due to the genetic differences between the two species. And more than that, there are genetic diseases for which it is impossible to create parallels in animals because the molecular mechanism that causes the disease in humans does not exist in animals, such as the fragile X disease that does not exist in mice. Another model for studying genetic diseases is cell culture taken from the patients themselves. But in many cases it is impossible to create a cell culture from the group of cells affected by the disease and grow it for research in the laboratory, as for example in a disease that affects nerve cells.
The models that exist today for the study of genetic diseases therefore make it possible to study only some of the characteristics of the disease and only in a limited way.
Production of embryonic stem cells for the study of genetic diseases in humans
In the innovative project that we set up in the in vitro fertilization laboratory at Lis Maternity Hospital, in collaboration with Dr. Rachel Eiges and Prof. Nissim Benvanisti from the Department of Genetics at the Hebrew University, we are producing embryonic stem cells that naturally carry the mutation for a known genetic disease in order to serve as a research model for the disease they carry . The stem cells are extracted from 5-day-old embryos (blastocysts) carrying genetic diseases, obtained from couples undergoing pre-implantation genetic diagnosis - PGD. Embryonic stem cells are pluripotent cells that simulate cells at the beginning of embryonic development, and in addition, they have the ability to divide endlessly, allowing them to be grown in culture over time. Also, it is possible to induce differentiation in these cells into almost all types of cells (nerve cells, muscle, etc.) and thereby also induce differentiation into those tissues affected by the disease. These sorted cells have the same genotype as that known from patients and it is likely that because of this their external expression (phenotype) will be very similar to the manifestation of the disease in humans. These cells therefore have great potential for the study of genetic diseases.
Since 1998, when human embryonic stem cells were produced for the first time, many studies have been published on the subject, some of which also use the stem cells to study genetic diseases. However, in the vast majority of these studies it is about cell lines with a normal genotype in which it is necessary to create targeted mutations by complex genetic manipulation in order to simulate the mutation typical of the disease being studied. The advantage of creating embryonic stem cell lines from preimplantation embryos affected by the disease is the fact that the cell lines carry the mutations naturally and the need for genetic manipulation is avoided. Moreover, the cell lines can also include mutations that cannot be created using molecular biology techniques, such as small missense mutations, point mutations, translocations and trisomies. Each of these cell lines serves as a model for a specific genetic disease and will enable the advancement of research on the disease for the purpose of understanding the mechanisms that cause it. They will also serve as a model for the development of drugs that will help in finding treatment and cures for the disease.
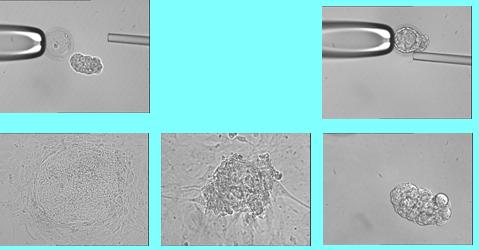
The research method
Embryos created in vitro are grown in an incubator for three days until they have eight cells. Then a single cell is removed from the embryo on which a pre-implantation genetic diagnosis, PGD, is performed, according to which it is determined which are the healthy embryos that will be returned to the woman's uterus in order to achieve pregnancy and the birth of a healthy child. The process of growing the embryos and the biopsy of the single cell is done by embryologists in an in vitro fertilization laboratory. The embryos diagnosed as sick and not used by the woman are incubated in the laboratory for about two more days and are used to produce embryonic stem cells. The cells are grown in culture under conditions that ensure division and inhibit differentiation. A series of splits allows the massive culture to obtain a large number of unsorted colonies and to establish a stable cell line over time.
Establishment of a bank of embryonic stem cells carrying various mutations
The pre-implantation genetic diagnosis project, from which the sick embryos are taken to create the cell lines, has been operating in the unit since June 2002. Today, the flow of referrals from carriers of genetic diseases who wish to undergo pre-implantation genetic diagnosis is large, and many have to wait several months for diagnosis. Dr. Mira Malkhov, head of the PGD field at the unit and Prof. Yuval Yaron, director of the prenatal diagnosis unit at the Genetic Institute established an established and reliable PGD system capable of diagnosing any disease for which the mutation that causes it is known. So far we have treated about 40 women and diagnosed about 20 different mutations in a single cell, some of which are very complex from a genetic point of view. Almost 20 women became pregnant in the unit after PGD and 8 of them have already given birth to healthy children, a fact that places us as the leading unit in the field in Israel.
Patient couples who undergo PGD to detect genetic diseases usually have a sick child in the immediate or extended family, and are therefore also highly motivated to contribute to the research of the disease they are carrying. At the end of 2004, for the first time in Israel, we received approval from a national ethical committee to produce stem cells from embryos diagnosed as carrying a mutation. During 2005, we established the system and asked couples undergoing PGD in the unit to donate the sick embryos to research. The alternative to donation is the termination of growth of embryos diagnosed as blue. However, our experience shows that the patients' response is extremely high, and so far everyone has responded.
So far we have produced several lines of embryonic stem cells that carry mutations for various genetic diseases. At the same time, we are working on the production of embryonic stem cells that carry additional genetic diseases with the aim of expanding the bank we have established, which is a reservoir of cells for the various diseases. The embryonic stem cells in our possession will be dedicated to research in cooperation with scientists and companies that study genetic diseases or that wish to perform a scan of potential drugs to cure or treat the disease.
Innovative results in research on the fragile X disease
The first embryonic stem cell line we produced carries the known mutation for the common disease called fragile X disease, and this allows us to study the molecular mechanism that causes the disease to manifest. This severe disease is the most common cause of mental retardation after Down syndrome (Mongolism). It is a genetically dominant disease whose carrier frequency in women is 1:150. Carriers have up to a 50% chance of giving birth to a sick child. In the gene responsible for the disease, FMR1, a dynamic mutation is possible. That is, the gene has a sequence of three nucleotides, CGG, that repeats itself many times. In healthy people the number of repetitions is between 5 and 55. The carriers of the permutation have between 55 and 200 repeats and they have a high chance that in the next generation another amplification will occur that will increase the number of repeats to over 200. In the event that such an amplification occurs the individual is sick, because a large number of CGG sequences causes the activation of a molecular mechanism that inhibits the expression of the FMR1 protein which is Has an important role in the development of nerve cells. The molecular mechanism that causes the increase in the number of CGG sequences and the transition from premutation to mutation is not clear. It is also unknown when exactly all this takes place.
There are several mouse models used to study fragile X disease, but none of them are optimal for studying the molecular mechanism of the disease. We produced a stem cell line, from embryos diagnosed by PGD as having inherited the allele that carries the permutation, we proved that the cells did have an increase in the number of repeats in the gene for the disease and they do display the mutation, with all its molecular characteristics exactly as found in Fragile X patients. We named the new cell line HEFX1. In the study, we showed that the line of embryonic stem cells that we produced divides in the laboratory without limitation and displays characteristic markers of unsorted cells. And we were able to show differentiation of the cells in the living body (in vivo) and in vitro (in-vitro) to form teratomas and Embryoid Bodies. The most impressive finding was when we followed the expression of the FMR1 gene in the cells. We showed that HEFX1 cells are unsorted, despite the presence of the mutation the gene is expressed at both the RNA and protein levels. The ability of embryonic stem cells to undergo differentiation, as we have shown, similar to the first stages of embryonic development, allows us to investigate the exact timing of the manifestation of the disease.
We hope that the embryonic stem cell lines that carry known mutations that cause severe genetic diseases will serve as a good research model that will enable the study of the pathophysiological basis of the disease and in the future will even help in the development of treatments and drugs for it.
Dr. Dalit Ben-Yosef is the director of the in vitro fertilization laboratory, and Professor Ami Amit is the director of the in vitro fertilization unit at the "Lis" maternity hospital in the Sourasky Medical Center in Tel Aviv (Achilov). Contact: dalitb@tasmc.health.gov.il
