This discovery could lead to the development of new synthetic elastic polymers
Scientists have succeeded in deciphering the structure of the protein that gives human tissues their elastic properties. This discovery could lead to the development of new synthetic elastic polymers.
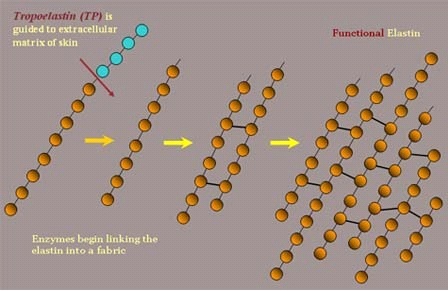
Researchers from the University of Manchester, in collaboration with scientists from Australia and the USA, used the most advanced methods to reveal the structure of tropoelastin, the main component of the protein elastin (Wikipedia). This protein allows tissues in humans, and other mammals, to stretch, for example when the lungs expand and contract during breathing or when blood vessels expand and contract during the millions of heartbeats.
The study, published in the scientific journal Proceedings of the National Academy of Sciences, reveals how evolution succeeded where engineers had so far failed to produce a molecule with near-perfect elasticity that would last a lifetime.
"All mammals rely on the protein elastin to provide their tissues with the ability to stretch and return to their original shape," said researcher Clair Baldock, from the University of Manchester. "This high level of physical ability required for elastin is successful and lasts much longer than any other elastic material created by man. "It is the coordinated collection of many tropoelastans that make up the elastin that gives the tissues their stretchability and it is this magnificent collection that helps produce elastic tissues of various types, such as arterial, lung and skin tissues.
"We were able to show that tropoelastin is a curved, spring-like molecule, with a "foot" area that helps bind it to cells. Stretching and relaxation experiments showed that the molecule has an unusual ability to elongate by eight of its original length and can then return to its original shape without any loss of energy, characteristics that make it an almost perfect spring."
The researcher adds and says: "Elastic components are used in a variety of applications, from clothing, vehicles, tissue engineering to space travel, so understanding the structure of tropoelastin and its elastic properties could enable the development of synthetic, elastin-like polymers, which have a variety of possible applications and many advantages."
One of the researchers explains: "Tropoelastin is a tiny protein similar to a "nano-spring" in the human body. Our body groups these nano-springs together to impart elasticity to a variety of tissue types, such as skin, blood vessels and lungs."

5 תגובות
Yaniv,
Even if it is not very complicated to decipher the chain of amino acids that make up the protein, it is not a particularly complicated thing (but more than you think: the RNA chain that is translated into a protein undergoes "editing" and cuts along the way), there remains the step of folding the protein into its final form and it is incredibly complicated. In order to decipher the spatial structure of the protein using X-ray radiation, the protein must be crystallized, and this is not a simple operation at all. Computational methods are also used to estimate the spatial structure of the protein, and you can participate in this here:
http://folding.stanford.edu/
The question is over:
Why is it so complicated to decipher the structure of a protein?
Is it that complex? Or are there technical problems to do this?
Thanks.
Wow
Surely the cosmetics industry will also have an interest in the matter
It's amazing, and looks like something with a chance for real reduction... elastic machines and all kinds of other things...!