Swiss and Russian researchers succeeded in creating single atoms of the two new elements, number 113 and 115. Will researchers be able to expand the periodic table with new elements in the future as well? The race continues
Yoram Ored, Galileo
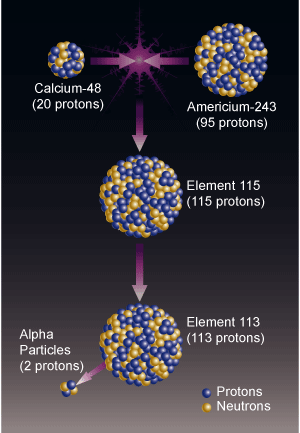
Swiss and Russian researchers succeeded in creating single atoms of the two new elements, which existed for fractions of a second.
Until 1940, uranium, with an atomic number of 92 (the atomic number is the number indicating the number of protons in the atomic nucleus) was the heaviest element known to the world of science. In the same year, the element neptunium, with atomic number 93, was discovered, followed immediately by the element plutonium, number 94. The race to discover super-heavy elements (that is, heavier than uranium) has continued ever since.
Recently, a joint team of Swiss and Russian scientists, working at the Russian Nuclear Research Center in Dubna (JNIR), succeeded in creating two more elements, with atomic numbers 113 and 115. First, scientists created the element with atomic number 115. They did this by bombarding a disk of americium (superheavy element number 95) with a beam of calcium atoms (atomic number 20). The fusion between the americium atoms of the disc and the calcium atoms led to the creation of an atom with atomic number 115.
Determining that such atoms were indeed created was not easy: the created atoms exist for only a tenth of a second. The scientists used a copper plate which they attached behind the americium plate. The copper plate absorbed all the americium atoms that were formed, and in this way the number of testable atoms increased.
Superheavy elements decay, among other things, through the emission of alpha particles, which are helium nuclei (the atomic number of a helium nucleus is 2). Each such decay causes a decrease of 2 in the atomic number of the element, or in other words, the creation of an element whose atomic number is 2 less. After the first decay of the new element, element 113 was formed, then roentgenium, followed by meitanrium, boehrium, and finally dubnium, which has atomic number 105. The scientists detected a pattern of five consecutive alpha emissions (meaning the atomic number decreased by 10), an occurrence that indicates its existence of the element with atomic number 115 and of that with atomic number 113 (which was created after the first alpha emission).
After five alpha particle emissions, 15 doubnium atoms were discovered, created as mentioned during the five-step decay. Dubanium is an element with a half-life of 32 hours, very long in terms of superheavy elements, a fact that facilitates their identification and the conclusion that two new elements were indeed formed.
And the race continues.
For information on the subject, visit the US Department of Energy website
