The research findings of a team of chemists may affect worldwide efforts to produce safer and cleaner nuclear energy and reduce the amount of radioactive waste
The research findings of a team of chemists may influence worldwide efforts to produce safer and cleaner nuclear energy and reduce the amount of radioactive waste.
The researchers used metal-organic frameworks (MOFs) to capture and remove volatile radioactive gas (iodine) from nuclear fuel waste. "This is one of the first attempts to use materials from this family to trap iodine," said chemist Tina Nenoff from the Department of Surface Sciences at Sandia National Laboratories belonging to the Lockheed Martin Corporation.
The discovery could be used for the reprocessing of nuclear fuel or for cleaning nuclear reactors after work accidents have occurred in them. One of the characteristics of nuclear energy is that fuel that has already been used can be reprocessed in order to recycle fissile materials and provide new fuel for nuclear power plants. Countries such as France, Russia and India reprocess used fuel. The process also reduces the volume of waste itself. "The goal is to find a method for the most selective separations that give rise to a situation of reducing the amount of nuclear waste transferred to the landfill," says the researcher.
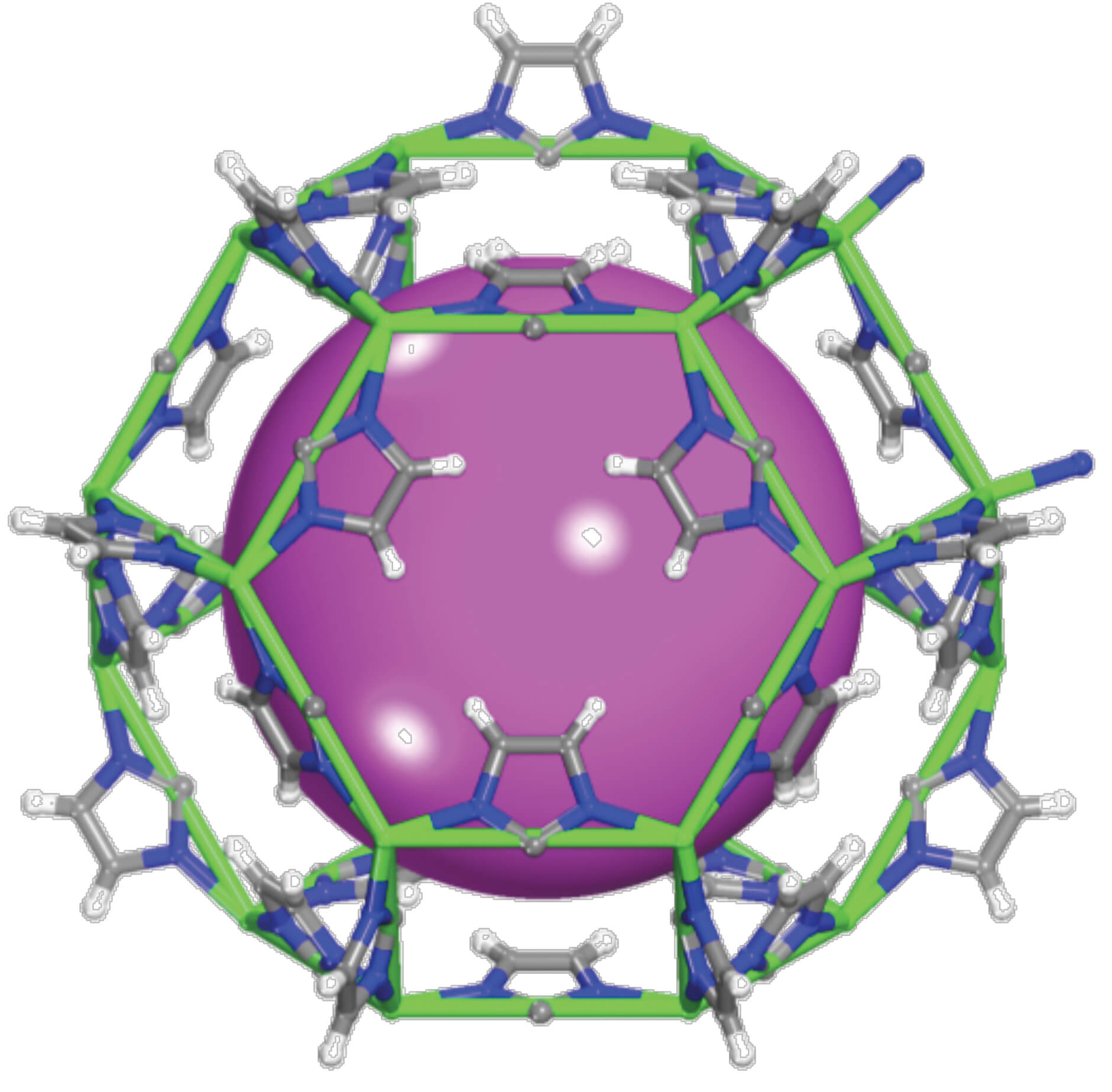
Part of the challenge in reprocessing is in the separation and isolation of radioactive components that cannot be utilized as fuel. The team of researchers focused on removing iodine, a substance whose isotopes have a half-life of 16 million years, from spent fuel. They studied known materials, including silver-rich zeolites, which are crystalline and porous materials with regular apertures, high surface area and high mechanical, thermal and chemical stability. There are zeolites capable of capturing and removing iodine from a stream of spent nuclear fuel, but they require the addition of silver in order to function properly. "The silver atoms bind to the iodine atoms to form the silver iodide salt," explains the researcher. "The zeolite contains the silver atoms in its pores and then these react with the iodine atoms to obtain silver iodide trapped inside the zeolite."
However, silver is an expensive material that is also an environmental nuisance, so the team of researchers tried to prepare materials without silver that could act similarly to zeolite as well as have a higher capacity for capturing gas molecules. They researched and tested how exactly the zeolite adsorbs the iodine atoms into it, then used the most essential components within it to arrive at the best material from the organometallic skeleton family, nicknamed ZIF-8. "We studied the structural properties thanks to which the previous material worked and copied them into the new and improved materials," says the researcher.
Organometallic frameworks are crystalline and porous materials in which a central core of metal atoms is linked to organic molecules through chemical synthesis. It is the choice of the type of metal and the type of organic molecule that gives rise to the unique finished chassis. The challenge was to find such a chassis with high selectivity for iodine atoms.
The researchers took the best features of the zeolite called Mordenite - its porosity, its large surface area, its stability and its chemical adsorption - and found a metallic organo skeleton capable of separating a certain molecule, in this case iodine, from a host of other molecules. The organometallic chassis and the iodine gas trapped in its pores can be injected into waste glass for long-term storage. The researchers were also able to create the finished product in stable tablet form, rather than powder form, which tends to disperse easily with any light breeze. A patent application has been submitted for this shape. The research findings were published in the scientific journal Journal of the American Chemical Society.
The news about the study

6 תגובות
Ernest,
You mean the radioactive materials from coal plants? I would assume they don't cause any damage but I'm not sure about that.
sympathetic
Do those radioactive materials emitted into space cause any damage?
Avi,
correction. Iodine has one isotope whose half-life is millions of years, iodine 129. The other isotopes have a half-life of fifty days, the longest of which has a half-life of 59 days. As far as I know, the short-lived isotopes are the isotopes created in reactor accidents.
Avi,
The half-life of radioactive iodine is of the order of days, not years, let alone millions of years.
sparrow,
Fission technology gives humanity a clean source of energy where no greenhouse gases are emitted into the air. For your information, a coal-fired power plant emits more radioactive materials into space than a nuclear reactor.
Personally, I hope that fission technology will pass from the world one hour earlier.
By Avi Luz
What do we do with the silver iodide or the other material?
It is a problem to bury these materials for a million years!