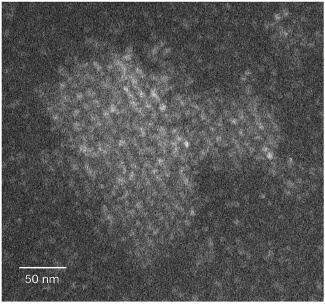
"The ferritin crystallized in a completely different way from the classical model: a perfect crystalline nucleus did not immediately form and kept growing, but rather a cluster of molecules characterized by a very low degree of order. This cluster gradually became denser and more orderly - first in the center, but over time the order spread to the edges as well"
Starting with our bones and ending with the cellular devices in the palms of our hands - crystalline materials occupy a central place in our world. However, for the most part, we are unable to predict which crystal will form, if any, from a certain material - and even the "classical" crystallization model is now in doubt. In a study recently published in the scientific journal Nature, Weizmann Institute of Science scientists were able to observe the formation of crystals in XNUMXD at the level of the individual molecule for the first time, and discovered a mechanism that helps explain crystallization cases that do not fit the classical model. The answer to the question of how crystals are formed will help to understand how order grows in our world out of disorder, and will allow in the future to develop medicines and many other crystalline substances for human well-being.
Perhaps it is not surprising that the crystallization process is so difficult to decipher, since in a certain sense it is contrary to common sense: usually in nature, just like in our wardrobe, a special effort is required to bring order to the "mess". How, then, do ordered crystals spontaneously form in a "messy" solution? According to the classic model for crystal formation, developed at the beginning of the 20th century, molecules that randomly collide with each other in a solution sometimes form bonds between them. At a certain point, the molecules are organized into a tiny crystal nucleus that later grows into a larger crystal - by adding molecules to its ordered structure. In the last 30 years, with the advancement of microscopy and other imaging methods, scientists in various laboratories around the world have begun to discover cases of crystallization that do not conform to the classical model. For example, in some cases, crystals were seen that started as a non-crystalline (amorphous) aggregate of molecules. These findings indicated that the classical model should be reconsidered, and raised questions about the exact crystallization process.

Prof. Boris Rivchinsky from the Department of Organic Chemistry and his group have acquired unique experience in recent years in observations of crystal formation. In the current study, they set themselves a challenging goal: to observe the crystallization of proteins - particularly large and complex organic molecules. "We wanted to approach the observations without prejudice - that is, without trying to confirm one theory or another - and the best way to do this was to track in XNUMXD the position of each of the protein molecules throughout the entire process," explains Prof. Rybchinsky. "From a technical point of view, in most organic substances, it is currently not possible to observe the crystallization process at the level of the single molecule, but we hypothesized that it is still possible to follow individual molecules of very large proteins."
Dr. Luther Hoven and Dr. Sharon Wolf from the Department of Chemical Research Infrastructures suggested using an imaging method known as cryo-STEM tomography - a method they previously developed with Prof. Michael Elbaum from the Department of Chemical and Biological Physics. As part of this method, the samples undergo vitrification (glazing), and immediately afterwards they are scanned from different angles using scanning electron microscopy (STEM). The method, combined with mathematical data analysis, allowed Dr. Hoven - along with Dr. Wolff and Dr. Haim Wiseman from the Department of Organic Chemistry - to reconstruct the position in space of each protein molecule and quantify the development of order in the samples. The protein that the researchers used for the experiment is ferritin - a protein that stores iron in various tissues of the body. The scientists scanned the ferritin samples after vitrifying the solution in which they crystallized at different stages of the process. When they located the position of each of the ferritin molecules in the samples, they were able to estimate the distances between them and their density, and measure their spatial arrangement compared to the perfectly ordered crystal. The findings revealed that the ferritin crystallized in a completely different way from the classical model: a perfect crystalline nucleus did not immediately form and kept growing, but rather a cluster of molecules characterized by a very low degree of order. This aggregate gradually secreted water molecules, so that the protein molecules in it got closer to each other, and gradually became denser and more ordered. The crystallized ferritin was first more ordered in the center of the aggregate, but over time the order spread to the edges as well.
These findings indicate that the classical model for crystallization is not necessarily wrong, but it only explains some of the cases. "It is possible that there is a sequence of possibilities for crystallization in nature - from the classical model to the non-classical process that we saw in Feritin," says Prof. Rivchinsky. "And it may also be that crystallization is always a gradual process, but it occurs at different speeds, and when order is formed quickly, it seems as if it was formed immediately, as described in the classical model."
Crystals to order
Not all crystals are created equal - even when it comes to crystals of the exact same material. For example, when crystals are produced in the pharmaceutical industry, a dozen different structures may be formed from the same molecules, but only one of them may be suitable for medical use. Similarly, when producing crystals for use in optoelectronic devices, controlling the crystal structure is essential to achieving the desired properties. Understanding these mechanisms in depth may help design custom crystals. For example, in the pharmaceutical industry, they will be able to produce from the beginning only crystals with the desired structure that will have the desired properties. Determining the structure in advance will also be very useful in the production of crystals for solar cells, cell phones, television screens and other devices. Specifically, protein crystallization is essential for the production of protein-based drugs, such as insulin, and is also important for deciphering the structure of proteins using X-ray crystallography.
The scientists were able to study ferritin crystals containing between 200 and 3,000 molecules only. For comparison, when deciphering using X-rays, only much larger ferritin crystals, containing hundreds of thousands or even millions of molecules, can be studied.
More of the topic in Hayadan:

One response
Is it possible to move a "handicapped" sign to a place that won't hide text?