Scientists from the Weizmann Institute examined a molecular junction with silver contacts, and showed that the nature of the interface between the contacts (electrodes) and the molecule dictates the mechanism that limits conduction through the molecule.

To test molecular conduction, scientists build electrical devices that consist of metallic electrodes with the molecule being tested between them. Typically, the molecule is attached to the electrodes via side chemical groups that maintain separation, so the molecule largely retains its properties. In this study, Dr. Tal and the members of his group chose to forgo the side groups, in order to remove the potential obstruction to the efficient passage of an electron stream. They used a device inside an empty chamber (vacuum), which cools a hair-thick metal wire to minus 269 degrees Celsius, then bends it, so that the wire tapers in the center to the thickness of a single atom, and finally breaks. In the space created between the two parts of the wire that end in an atomic tip, the molecule under consideration is captured from a large number of molecules that were released into the cell space, and this is how the molecular junction is formed.
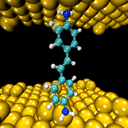
"We create contact between the molecule and the metal, and the interface creates new properties," says Dr. Tal. "For example, about a year ago we discovered that if you put a molecule of oxygen between two nickel electrodes, the molecule breaks up into atoms that bridge the electrodes. The created atomic junction allows the passage of electrons with spin in one direction only, a very desirable feature in the field of spintronics - spin-based electronics. This is an example of a new behavior created at the interface between different materials, which does not characterize each material separately."
To obtain high conductivity in the molecular junctions, molecules are used in which some of the electrons are free, and can be found anywhere on the molecule. The molecules were connected directly between two metallic electrodes so that the electron orbits on the molecule coalesce to a certain extent with those of the mobile metal electrons. In conduction there is a competition between two opposite trends: on the one hand, the longer the molecule, the more its energy levels match those of the metal, and therefore the conduction is better; On the other hand, as the molecule is longer, the orbit of an electron on the molecule is spread over a longer structure, so that the extent of its overlap with the metal is lower, which limits conduction.
In the study, which they carried out in collaboration with the research groups of Prof. Ferdinand Ebers from Germany and Prof. Colin Neckles from the United States, the scientists found that in a molecular junction with silver electrodes, when the molecule is relatively short, the first direction is dominant, and the conduction increases as the length of the molecule increases, up to a saturation point in The trends cancel each other out. On the other hand, in a junction with platinum electrodes, the fusion of the molecular electron orbits with those of the metal is very effective, and the molecule behaves as a continuation of the metal, so that the conduction through it is saturated and similar to that of a metal, that is, it is not sensitive to differences in the electronic structure of molecules of different lengths.
"We were able to investigate for the first time the upper limit of conduction at a molecular junction, and we showed that the nature of the interface between the electrode and the molecule dictates the mechanism that limits conduction through the molecule," says Dr. Tal. "We are interested in learning how to control electrical conduction at the edge of electronic miniaturization. Because of the structural richness of molecules, their use as an electronic device allows us to search for general principles for controlling electrical conduction on a nanometer scale - principles that can also be useful in other nanometer systems."

One response
Thanks for the report. It is also useful to know where the (scientific) article was published