Molecular structures of a closed cage in nanometer dimensions have been known for years - first structures with pure carbon were known and later Prof. Reshef Tana's discovery of a panorama of inorganic components was published. The theoretical basis for why these unusual molecular cages form was discovered later
A new and unprecedented closed molecular cage nanostructure, which is produced using highly concentrated sunlight, was recently revealed by a team that includes Professors Jeffrey Gordon and Daniel Feuerman from Ben-Gurion University of the Negev, the research group of Prof. Rashef Tana from the Weizmann Institute of Science and Dr. Andrei Anyishin from the Ural Federal University in Russia.
The findings of the research that reveals the nanostructure of a closed molecular cage was published in the latest issue of one of the most respected journals in the field of nanotechnology - DHW Nano .
Molecular structures of a closed cage in nanometer dimensions have been known for years - first structures with pure carbon were known and later Prof. Reshef Tana's discovery of a panorama of inorganic components was published. The theoretical basis for why these unusual molecular cages form was later discovered - they were found to exhibit surprising and unexpected electronic, mechanical and chemical properties.
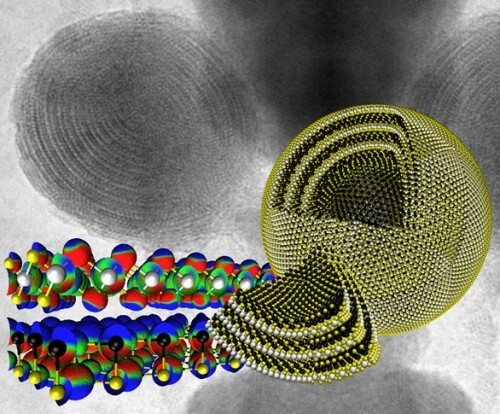
But so far no closed-cage nanostructures, which consist of the alternating layers of different chemical components, have been proposed or attached, each of which on its own will not be successful, but together, as a superstructure of "unsuccessful" components, they will succeed. In this case, the two ingredients were lead sulfide and tin sulfide.
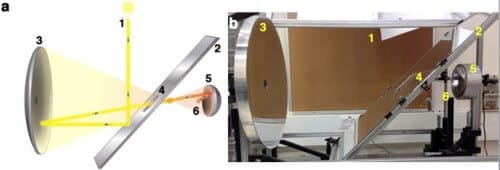
This experimental success story was achieved thanks to an advanced solar furnace, designed and built by Professors Gordon and Feuerman in their laboratory at the Sde Boker campus of Ben-Gurion University of the Negev (in the Alexander Yersin Department of Solar Energy and Environmental Physics at the Jacob Blaustein Desert Research Institute). It has been proven that highly concentrated solar radiation is a key element in the creation of a unique reactor, which leads to the fusion of these unsuccessful individual nanostructures. The reactor needs temperatures that reach 3,000 degrees Celsius over time, relatively large irradiation areas, a very hot ambient incandescent environment and sharp gradients.
Thanks to complex molecular calculations, contributed by Dr. Andrey Anyishin, it is possible to explain, quantify and present the polar forces between the various molecular slices of tin sulfide and lead sulfide, with the goal that they will play a central role in stabilizing unsuccessful superstructures that are composed of layers, including the range of particle sizes (100 -20 nm) that was discovered in the experimental products.
These groundbreaking findings predict future research, where it will be possible to identify a rich range of unsuccessful nanostructures built in layers, and fuse them. These studies are currently being planned in coordination between Rashef Tana, Jeffrey Gordon and Daniel Feuerman.
Because of the unexpected existence of such nanostructures, it is still too early to address the extraordinary properties and applications they can offer. However, the known properties of lead sulphide and tin sulphide, each individually, suggest that their disordered nanostructures can reveal interesting optical absorption of light, which could lead to applications in photocatalysis and photodetection.