The children suffer from congenital heart defects. The catheter implantation involves minimal invasiveness
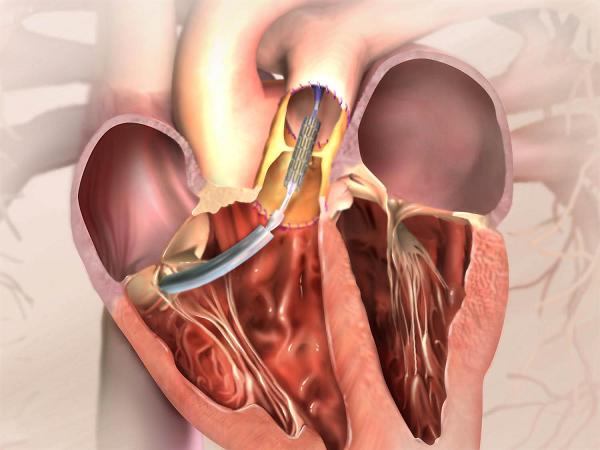
This week, several catheterizations for pulmonary valve implantation in children suffering from congenital heart defects were successfully performed at the Schneider Pediatrics Center of the General Group. Pulmonary valve transplantation has been performed to this day in open heart surgery with a heart-lung machine. The catheter implantation, on the other hand, involves minimal invasiveness: entering the groin vein while the patient is anesthetized and the heart is working normally. The valve intended for transplantation is sewn to a metallic support (stent), folded and worn on a balloon. It is advanced under mirroring to the place intended for transplantation. There, the balloon is inflated in a controlled manner until the valve is securely fixed and then the balloon is deflated and removed from the body. At this point the valve goes into immediate action. The recovery from the transplant process is fast, and the patient can go home the next day and return to normal activities within a few days. Therefore, and especially in light of the fact that most of these children have undergone one or more heart surgeries in the past, the catheter implantation represents a major revolution in the treatment of valvular problems in general and children's malformations in particular, both from a purely medical point of view and in terms of discomfort and risk to the patient.
Congenital heart disease is the most common birth defect in the world, its incidence is about 0.8% of all newborns. About 20% of those babies are born with defects that disrupt the flow of blood from the right ventricle to the pulmonary artery. People who suffer from a birth defect that disrupts the flow of blood from the right ventricle to the pulmonary artery tire very easily, because the heart reaches an overstrain trying to pump blood to the body's organs.
This type of defect usually requires open heart surgery at a very young age to implant a foreign valve to repair the defect. However, with the growing process of the child, the foreign valve ceases to fit him, also the duration of the functional life of such a valve is limited. As a result, most patients with this type of defect have to undergo several open heart surgeries during their lifetime to replace the valve. Until now, many children born with a pulmonary valve defect had to undergo between 3-4 open heart surgeries to correct the problem by the time they reached the age of ten. The Heart Institute at the Schneider Pediatric Center, headed by Dr. Einat Birak, specializes in the treatment of congenital heart defects, and over 400 surgeries and 500 catheterizations per year are performed by a multidisciplinary team of experts that includes cardiologists, surgeons, anesthesiologists, and intensive care physicians plus Adequate paramedical teams. The institute was chosen by Medtronic, the manufacturer of the "Melody" valve, to be one of about 40 centers in the world and the only one in Israel where the innovative technology is used. Adding this technology to the range of treatments currently available at the Schneider Center in the field of congenital heart defects places the hospital among the leading centers in the world in this field. The catheterizations were performed by Dr. Elhanan Bruckheimer, director of the catheterization unit at the Heart Institute, and Dr. Lee Benson who came especially from the Hospital For Sick Children in Toronto. Earlier, Dr. Birk and Dr. Bruckheimer went for training at the well-known Great Ormond Street Children's Hospital in London to learn the indications for the method and ways of applying it.
A "Melody" pulmonary valve inserted through a catheter was developed by Medtronic in collaboration with cardiologist Prof. Philip Bonhoeffer, head of the Heart Institute at the well-known Great Ormond Street Children's Hospital in London and a pioneer of catheter valve technology. The valve is made from the jugular vein of a cow, and works in the same way as the heart's natural valve. It ensures the flow of blood from the right ventricle to the lungs, and eventually to the rest of the body's organs. A unique conduction system is added to the valve, which includes a catheter (a long tube) used to transfer the melodic valve through a vein in the leg to the correct location in the heart. The catheter contains a "balloon-in-balloon" system that expands and places the valve at the desired point. Prof. Bonhoeffer implanted the first valve in September 2000. To date, the Melody has been implanted in over 400 patients worldwide.
Dr. Einat Birak, Director of the Heart Institute at the Schneider Pediatric Center: "Pulmonary valve replacement through a catheter is safe, quick and convenient. It allows us to avoid the procedure of re-opening the chest and open heart surgery. The possibility of replacing a valve with a catheter, which seemed imaginary only a few years ago, has become a reality. I would like to express my great appreciation to the general health services management for the efforts and willingness to introduce this new technology."
Dr. Elhanan Bruckheimer, director of the catheterization unit at the heart institute at the Schneider Center: "This breakthrough is exciting because it proves once again that today's impossible can be turned into tomorrow's standard treatment. There are currently developments for many methods to treat valves during catheterization both for congenital heart defects and for heart problems in adults."
"Mestam Melodi is an example of Medtronic's commitment to developing scientific knowledge about structural heart disease and placing it at the center of the stage" states Judit Gal, CEO of Medtronic Israel. "This focus illustrates our adherence to the goal of providing innovative valve technology that meets the needs of the patient for whom a solution has not yet been found. When we give the doctors less invasive options and in this way prevent the need for even one operation for a patient, we immeasurably improve the quality of life of that patient. I am very happy for the opportunity to collaborate with the Heart Institute at the Schneider Center on this exciting topic. As an Israeli, I am proud that the company's management chose it as one of the first operating sites for the technology alongside a limited number of leading centers in the world, and hope that this is a sign of things to come."
