Surgery to shorten the intestines eliminates the disease, and new evidence indicates that the intestines - and not just insulin - may be responsible for the disease
- Forty-five medical organizations now recommend weight loss surgery as a standard treatment option for type 2 diabetes.
- Many clinical trials show that surgery controls diabetes better, faster and for a longer period of time than dietary changes and drugs.
- The success in the surgeries indicates a connection between diabetes and the intestines. Surgery can be successful because it changes the gut hormones, the bile acids, the gut bacteria population or removes the cause of the disease.
About two decades ago when I started my internship as a surgeon, I longed to treat tumors, gallstones, hernias (hernias) and everything that was within reach of the surgeon's knife in my hand. Surgery appears to be a straightforward solution to some serious problems.
Type 2 diabetes There wasn't one of them. Surgeries focus on a specific body part, but doctors know that diabetes causes damage to many organs and involves a failure to effectively use the blood glucose regulating hormone - insulin. Clearly, this was not something that could be reduced or eliminated through surgery.
But one afternoon in the summer of 1999 there was a radical shift in my perspective on diabetes and my career.
It was when I had just moved from Italy to New York City thanks to a research grant I received to specialize in minimally invasive surgery at the Icahn School of Medicine at Mount Sinai Hospital. I was in the library trying to read up on some technical aspects of sham surgery Tilting of the bile and pancreas, when I encountered something strange. The surgery is intended for people who suffer from severe obesity. The process, which involves cutting out a part of the stomach and connecting it to the lower end of the small intestine, significantly shortens the path that the food travels through and bypasses the parts of the small intestine that absorb the food and calories and thus causes people to lose weight. Many of those operated on suffered from type 2 diabetes which is accompanied by obesity. But what struck me was that already a month after the operation, the sugar level in the blood of the operated people dropped to a normal level. They still haven't lost weight, they ate without restriction of sugar and calories and didn't take any diabetes medication. And yet most of them remained diabetes-free years after the operation.
I was filled with wonder. How can surgery correct blood sugar problems in a disease that is written in all textbooks as chronic, progressive and irreversible? It can be managed but it should not disappear.
I racked my brain for an explanation and remembered that the small intestine produces hormones that stimulate the pancreas to produce excess insulin. Could it be that a surgical change in anatomy affected these hormones in a way that restored glucose metabolism? Or is the intestine responsible for other mechanisms of the disease that surgery could correct? If so, surgery can treat diabetes, and understanding how it does so can provide clues about the elusive mechanism for developing diabetes.
At that time, in the late 90s, we were just beginning to realize that we were already in the midst of a diabetes epidemic that continues to this day. The latest estimate by the International Diabetes Association and the World Health Organization is that at least 415 million people in the world suffer from this disorder and the number is expected to increase to about 650 million by 2040. (90% of them have type 2 diabetes and the rest, another form of the disease, type 1 diabetes which results from the fact that the pancreas does not provide enough insulin.) Finding the cause and cure for type 2 diabetes will save the lives of millions of patients.
After a sleepless night, shaken by the possibilities, I went in the morning to the surgeon in charge, Michel Gagner, and shared the idea with him. He thought I was onto something. Together we approached the officials of the medical school to get permission to conduct a clinical trial on humans, to check if the surgery can improve the condition of diabetes more than the routine treatments even in people who do not suffer from severe obesity. Our proposal was rejected not only then but also in the following months.
We were disappointed by the rejection, although we were not surprised. For hundreds of years diabetes has been treated with diets, pills and injections. Since they assumed that the cause was a problem with the activity of the cells in the pancreas that produce insulin and also a problem with the way the body reacts to the hormone, then surgery, including the removal of parts of the intestine as a remedy, seemed heresy and a stupid risk.
Two decades later the apostasy became common opinion.
To date, dozens of animal studies and at least 12 randomized controlled clinical trials involving hundreds of people have tested the possibility that a surgery first developed as weight-loss surgery is effective in treating type 2 diabetes. They all show that lowering the surface area of the gastrointestinal (GI) tract has a much greater effect on diabetes than the prevailing treatments. . And this is not simply a result of losing weight. In many patients, blood sugar levels returned to normal within weeks, long before fat levels or pounds began to melt away. In general, about 50% of patients recover from diabetes after surgery, and some remain that way years later. The rest of the operated show a marked improvement in blood sugar control and can radically reduce their dependence on insulin or other drugs.
The evidence is so solid that last year 45 medical associations recommended gastric bypass surgery as a standard option for treating diabetes even for moderately obese patients. Moreover, knowledge of the mechanisms by which intestinal surgery affects glucose metabolism encourages the development of nonsurgical approaches that target the small intestine.
Collecting facts
In the weeks after the discovery that jumped me in the library, when our proposals to test surgeries on people with diabetes were rejected, I delved deeper into the medical literature in search of evidence to support my idea. I learned that doctors saw an improvement in diabetes after gastrointestinal surgery as early as a century ago. In 1925, an article in the Lancet described the almost overnight disappearance of excess sugar in the urine, a symptom of diabetes, after one gastrointestinal surgery for the treatment of a gastrointestinal ulcer. Similar observations became more common after these surgeries became popular as a treatment for severe obesity in the mid-50s. In the 20s and 80s, many reports noted the positive effects of this type of surgery on diabetes, including the landmark study by surgeon Walter Forris and his colleagues from East Carolina University, which included more than 90 patients and whose title explicitly declared:who would believe? Surgery is the most effective treatment for adult diabetes. "
Despite all these compelling observations, surgery is not considered a serious treatment for diabetes on its own. A major stumbling block was that many doctors believed that the positive effects on diabetes did not come from the surgery itself, but from the weight loss.
Resolving this debate in some way became important to Genger and me after we were unable to start clinical trials. I therefore turned to rats to investigate whether surgery that alters the digestive tract could directly affect glucose metabolism independent of weight change. I moved to the European Institute for Remote Surgery in Strasbourg, France. There, my colleague and I took lean rats with type 2 diabetes and performed a bypass operation on the duodenum and a large part of the small intestine, an experimental operation aimed at shortening the intestine without changing the size of the stomach. (The idea was to prevent mechanical eating restriction.) After the surgery, the rats showed an improvement in glucose metabolism whether or not there was a change in their body weight and the amount of food they consumed.
An impressive rate of 89% of diabetic patients who were operated on did not need insulin five years after the operation.
Other researchers have reinforced these findings by using such a bypass or other procedures in different animal models. Then, at the beginning of the 21st century, it was also demonstrated in humans. In the last decade, about 12 randomized clinical trials were conducted, and all of them showed similar results. In one of these studies Galtrude Mingron, from the Catholic University of Rome, together with me and other colleagues, we showed that five years after the operation in 38 patients, more than 80% showed a complete regression of the disease or were able to maintain the balance of blood sugar levels with the help of small amounts of drugs or With the help of diet and exercise. Data from another experiment led by Philip Schuder at the Cleveland Clinic, where 96 patients were operated on, showed that although about 45% of the patients needed insulin before the operation, after that, an impressive rate of 89% did not need the drug five years later. A large study of obese Swedish citizens showed that surgery can also reduce complications of the disease, such as heart attacks, strokes and diabetes-related mortality, more than conventional treatments. The safety of these procedures is comparable to that of any other normal surgery, including gallbladder surgery, or surgery for hysterectomy, which are considered low-risk interventions. Cost calculations show that the price of surgery (which costs $20,000 to $25,000 per procedure in the US) balances out within two to three years due to the reduction in expenses on diabetes medication.
The intestine as a focus for treatment
Why does surgery work so well? No one knows yet, but the digestive tract appears to be a key player in normal glucose metabolism and diabetes-related disorders. There are at least five ways in which the intestine can exert such an effect: through hormones, bile acids, molecules that transport glucose out of the intestines, bacteria that live inside the intestines, and neural circuits.
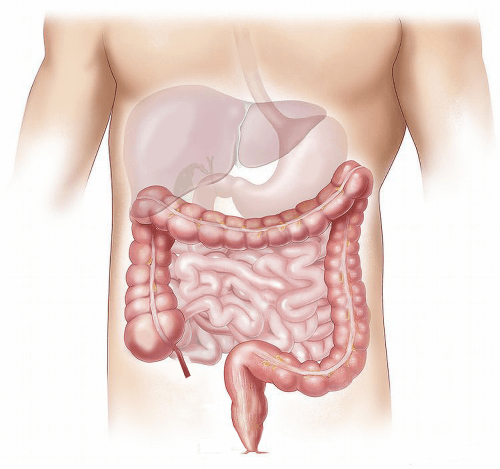
The walls of the digestive tract have cells that respond to nutrients and other stimuli by releasing hormones. These substances in turn stimulate the secretion of insulin from the pancreas or affect feelings of hunger and satiety. Changes in the anatomy of the digestive tract following surgery shorten the time it takes for food to pass through these cells and thereby reduce contact and irritation in some sections of the path. This also means that more food is available in the subsequent segments of the digestive tract. The end result is an increase in the levels of certain hormones and a decrease in the levels of others.
Studies conducted by David Cummings from the University of Washington in sick humans showed that gastric bypass surgery suppresses the levels of the hunger-inducing hormone - ghrelin - in the bloodstream, which is also known to regulate the entry of glucose into a certain type of cells. Carl W. Le Roux, now at University College Dublin, and other investigators have demonstrated that the so-called roux-en-Y And similar procedures boost levels of other hormones called incretins that increase insulin production.
Bile acids, another type of molecule that regulates energy use in the body, are also affected by GI weight loss surgery. Bile acids, known to many for their role in digesting food, also enter the bloodstream and signal to receptors on cells in various tissues and organs. The signals cause the cells to accelerate the use of fats and glucose. Gastric surgeries can increase the levels of bile acids in the blood circulation and thus help the cells obtain glucose from the blood. Studies also show that bile acids can prevent the accumulation of cells from the immune system, called macrophages, in fat tissue. A reduced presence of macrophages reduces inflammation and insulin resistance that are hallmarks of obesity and type 2 diabetes.
Surgery can also affect another mechanism that contributes to diabetes: molecules that transport glucose. During digestion, food particles break down in the intestines and glucose is produced. Glucose moves through the walls of the intestines and enters the bloodstream with the help of those transporter molecules. A high concentration of sodium is required for the molecules to function properly. But in some types of gastric bypass surgery, food-conducting segments of the intestines are rerouted in a way that bypasses their main sources of sodium - the digestive juices of the bile and pancreas. Without sodium, the activity of the molecules that transport glucose slows down considerably, thus improving blood glucose control by reducing the sharp increases in glucose after a meal.
Gut bacteria can also play a role. The digestive tract harbors trillions of microorganisms. Certain types help the body to extract energy from food and produce substances that reduce inflammation and insulin resistance. Since GI surgeries change the acidity of the gut and the amount and chemical composition of nutrients in the gut, they can change the local bacterial population. Lee Kaplan from Harvard Medical School and his colleagues have shown that this phenomenon can affect metabolism. They began gastric bypass surgery on a group of mice. A few weeks later, the researchers transplanted populations of gut bacteria from these mice into non-operated mice whose bacteria had been destroyed. This second group was fed a high-fat diet. They gained less weight and improved their metabolism much compared to rodents in which bacteria from mice that did not undergo the surgical procedure were transplanted.
Another known effect of the surgeries is on neural circuits that are related to metabolism. Such a circuit, for example, goes from the intestine to the brain along the vagus nerve. It allows the small intestine to sense tiny amounts of digested food and inform the brain, which in turn suppresses glucose production in the liver and thus generally lowers blood glucose levels. Tony Lam of the University of Toronto and his colleagues conducted experiments in rodents and showed that GI bypass surgery increases activity in this type of food sensing mechanisms.
Finally, it is possible that the surgery can eliminate some of the insulin-blocking mechanisms that can cause diabetes. The origin of the theory is in insulin-stimulating hormones - the incretins. These hormones need a buffer mechanism, otherwise they will flood the body with insulin after every meal. In such a case, we should suffer from low levels of blood sugar (hypoglycemia) after a meal, due to a surge of insulin that will remove the glucose from the bloodstream. Since people do not routinely go into a coma caused by low glucose after meals there must be something blocking the incretins. But if that blocking mechanism were to act to an extreme, it could suppress the body's response to insulin - in other words, cause type 2 diabetes. Such substances that I call "anti-incretins" have not yet been definitively identified, but some suspects have begun to emerge.
Gut hormones like Somatostatin-28 andgalanin Reduce insulin secretion in rodents. And there is more.
In 2013, Mingron and her colleagues isolated a group of unidentified proteins in a segment of the digestive tract of diabetic mice. When they injected the proteins into non-diabetic mice, they developed severe insulin resistance. (The proteins also acted the same way when injected into normal human muscle cells grown in the lab.) I believe that gastric bypass surgery can reduce the amount or availability of these anti-incretin insulin blockers and restore the normal balance of the body's metabolism.
Whatever the exact mechanism, these and other observations point to the digestive tract as a source of diabetes. Defective activity of the mechanisms triggered by food in the intestines can also explain how a worldwide increase in fatty and high-sugar foods in recent years, and an increase in the general availability of food in many countries can cause the spread of the epidemic.
Anti-diabetic devices
Although surgery can be a powerful medicine, it can never be a mass solution to a common problem. It requires hospitalization, well-trained staff, and the level of risk associated with using a surgeon's knife on a patient's body. Less invasive drugs are needed. At least one may already be available: a small sleeve that can be inserted into the intestines through the throat and stomach.
The idea is to cover the The duodenum, that segment of the digestive tract immediately after the stomach. This is the section where the bile and pancreatic juices first mix with the partially digested food and change the chemical properties of everything that continues down the intestines. Therefore, this one key point can affect the downstream digestive tract and most of the mechanisms of glucose control that I have described.
In a series of experiments, my colleagues and I blocked the duodenum in diabetic rats by inserting a flexible silicone tube that allowed nutrients to pass through this section. These substances did not come into contact either with the cells in the walls of the duodenum or with the bile juices. Blood glucose control improved markedly. But then we punctured the tube so that the nutrients leaked out. This change sabotaged the antidiabetic results.
There are already flexible plastic sleeves that protect the duodenum in humans. They were developed to mimic the effect of gastric bypass surgery without surgery, and have already been approved for clinical use in Europe and South America. In patients who underwent this procedure, there was a marked improvement in the symptoms of diabetes. There is also a newer approach, now in human clinical trials, where doctors slide a balloon attached to a device down the throat into the duodenum. They fill the balloon with hot water to burn cells that normally react to food ingredients. Early tests have shown promising results for type 2 diabetes, and more studies are on the way to examine long-term effects of the treatment.
This is not the first time in medicine that surgery paves the way for other types of treatments. This is not even the first time with diabetes. In 1889 g Oscar Minkowski dogs to develop diabetes by removing the pancreas, thus providing the crucial clue that led the Frederick Banting וCharles Best for the discovery of insulin in 1921. About a century later, the success of the surgeries highlighted the digestive tract as a target for new approaches to treating diabetes, approaches that I hope will help patients as much as, or even more than, insulin injections.

2 תגובות
Hello, how much does such an operation cost?
A doctor is a surgeon - and does not know enough about the functions of the human body!!!! Most of the absorption of sugar in the body is done in the duodenum. Its minority - further down the intestine (long starches whose conversion to glucose is a slower process). It is therefore clear and trivial that if you block the duodenum from "seeing food" - then it will not absorb glucose, and "miraculously" the glucose levels in his blood will drop. I suggest to the doctor who wrote the article to go back to school, beyond the fact that diabetes is a simple disability to treat (I have type 2 diabetes) - to eat only the appropriate foods. What is called in the vernacular "shut your mouth". Duodenal and intestinal bypass surgery to treat diabetes - sounds like the idea of a crazy professor.