How did Dan Shechtman, who this week (Saturday) receives the 2011 Nobel Prize in Chemistry, create a new and surprising scientific field
In 1984, Dan Shechtman from the Technion stunned the world of science, especially those involved in the fields of materials science, chemistry and solid state physics, by publishing the results of an experiment he had conducted two years earlier, while on sabbatical at the American Standards Institute. Shechtman prepared an aluminum solder that contained about 14% manganese using rapid solidification technology, which means cooling the liquid solder at a rate higher than a million degrees per second. After cooling, Schechtman examined one of the tiny crystals that formed using a transmission electron microscope. In this technique, the electrons passing through the crystal create a diffraction pattern with an orderly pattern that reveals the symmetry of the crystal. Crystals, according to their classical definition, which was accepted until then, are structures in which the atoms, molecules or ions that make them up are packed in an orderly manner and have a three-dimensional pattern which repeats itself. It is easy to prove mathematically that such structures can have twofold, threefold, square or hexagonal symmetry, but can not Have pentagonal symmetry or order 7 or higher.
However, the diffraction diagram found by Shechtman clearly showed icosahedral symmetry (symmetry of order 10) and that the tiny crystal also contains pentagonal symmetry - a result that completely contradicted the definition of the crystal because it does not allow for long-term periodicity. Nevertheless, Shechtman's diffraction diagram determined that the studied crystal has an incredible combination of "forbidden" pentagonal symmetry and an ordered structure! It didn't take long and crystals with similar symmetry were also discovered in other fuses prepared using rapid solidification technology that creates crystals that thermodynamically are considered unstable phases. Today there are researchers who claim that it is possible to grow thermodynamically stable crystals with such symmetry in normal production processes, and not necessarily by rapid solidification.
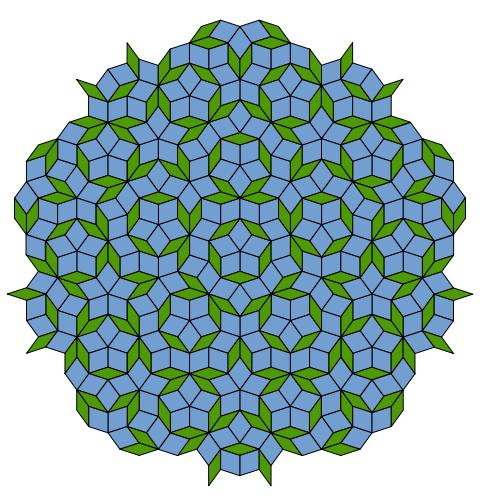
Immediately afterwards, a theoretical explanation was also found for such a crystalline structure, which has long-range order but no regular periodicity. The explanation is based, among other things, on a mathematical discovery by the British physicist Roger Penrose, who showed how it is possible to lay out a plane with tiles of a certain shape and with a certain regularity and obtain pentagonal symmetry without cycles (Penrose's discovery was published for the first time in Scientific American in Martin Gardner's famous mathematical entertainment column - the editors) . Five weeks after Shechtman's article was published, physicists Dov Levin and Paul Steinhardt coined the name "quasi-crystals" (literally: almost like crystals) due to the fact that, despite having long-range order in these structures, they do not have a periodic structure like classical crystals. Following this, the International Union of Crystallography changed the definition of the crystal to the experimental definition "a solid with a discrete diffraction pattern".
Dan Shechtman broke through to a new and fascinating science that stretched the limits of the imagination and knowledge of those involved in crystallography all over the world. The study of quasi-crystals has developed greatly in the years since the discovery. Today, in many universities around the world, researchers are engaged in growing and deciphering the structure of quasi-crystals and their approximants - crystals in which the atoms are organized in pions (polyhedrons) identical to those in quasi-crystals but organized in a cyclic manner.
The world of science had a hard time accepting Shechtman's surprising discovery, but almost 20 years later it awarded him the most honorable sign of appreciation: the 2011 Nobel Prize in Chemistry.
_______________________________________________________________________________________________________________________________________________________________
About the authors
Doctor Louisa Meshi and Professor Adin Stern are researchers in the Department of Materials Engineering atBen Gurion University in the Negev.

