In a research collaboration between the Hebrew University and the University of California at Berkeley, new insights were obtained for the first time on the mode of activity of chemical catalysts used to create fuels, plastics and fertilizers. The good news for consumers: in the future it will be possible to produce more efficient and environmental catalysts that will help reduce air pollution and lower production costs.
Most of the industrial materials that surround us and are essential to our daily functioning in the 21st century, such as fuels, plastic products or fertilizers, do not come as such directly from nature, but undergo chemical processes that change them to suit human needs.
In order for these chemical changes to be carried out effectively and to successfully transform a material from its raw form in nature into fuel for the car or fertilizers for the garden, catalysts are used. The catalysts mostly consist of tiny metal particles, for example platinum, iron, palladium or silver, with a nanometer size (billionths of a meter), which allows maximum utilization of their chemical properties.
An example of the use of such catalysts is, for example, in the process of producing fertilizers for agriculture. Most fertilizers are currently produced in an artificial process that uses iron particles as catalysts. The iron particles cause hydrogen molecules and nitrogen molecules, which normally do not react with each other, to break down and produce a new substance - ammonia, which is the main raw material in the creation of fertilizers.
Another example is the "catalytic converter" present in most cars on the market. Using nanometer-sized metallic particles that contain palladium and platinum, the toxic gases emitted from the car's exhaust in the process of burning fuel change their composition and become non-toxic or less toxic gases, thus helping to reduce the process of air and environmental pollution.
Although catalysts have been used to create various substances in the chemical industry for more than a century, there are still many details related to the process and how they work that are unknown to the scientific community. The reasons for these gaps in knowledge stem from the small size of the particles, which makes it impossible to examine and map the chemical reaction on the surface of a single particle. As a result, the ability to design and build more efficient catalysts to meet current energy needs is limited.
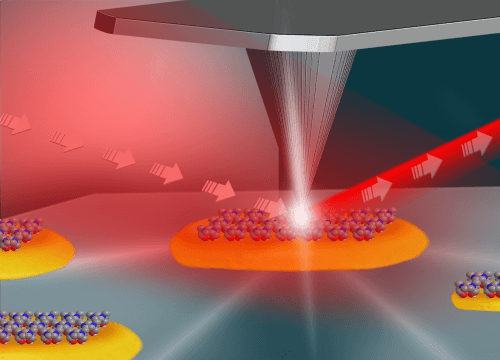
In a first-of-its-kind study conducted under the leadership of Dr. Elad Gross from the Institute of Chemistry and the Center for Nanotechnology at the Hebrew University and Professor Dean Tost from the Institute of Chemistry at the University of California at Berkeley, new insights were gained regarding the nature of the activity of chemical catalysts. For the first time in the world, chemical activity was observed on the surface of individual platinum particles. These particles are similar to the particles that make up the catalysts in the chemical industry.
In the study, the researchers used infrared light with a very high brightness (a million times brighter than the sun). The researchers focused the bright light beam towards a thin needle whose tip is 20 nanometers in diameter. This needle was used as a kind of "antenna" that focuses the light in the area close to the surface of the particle and thus made it possible to pinpoint the molecules on the surface of the nanometer particle. By scanning the particle with the needle, while irradiating with infrared light, it is possible to diagnose where and under what conditions the chemical process occurs on the surface of the particle.
The researchers observed that the chemical activity of the catalysts is concentrated at the edges of the particle, however, less chemical activity was observed in the center of the particle. The reason for the differences lies in the different nature of the platinum atoms in the center of the particle, which is flat and ordered, compared to the edges of the particle which are less ordered and uneven. Metal atoms that are on the surface of the flat part of the particle are surrounded by atoms that are identical to them and will therefore have a limited ability to interact with external molecules. On the other hand, atoms that are at the edges of the particle, in areas where there is a lack of uniformity at the atomic level and they are not completely surrounded by metal atoms, will be able to create a stronger interaction with external molecules and lead to the occurrence of a chemical reaction that will turn the molecule into the desired product.
The researchers' conclusions confirmed a well-known claim in the world of chemical catalysts that regions that are not perfect, and in which there is a high density of atomic "defects", are the most chemically active.
Dr. Elad Gross, one of the leaders of the research said: "The results of the research proved, for the first time, that it is possible to expect increased chemical activity in areas with a high density of defects on the surface of individual particles. The research conclusions will enable better future planning of chemical catalysts, and the production of more efficient catalysts that are capable of turning materials found in nature into products with high added value."
The implications of the research are that it will now be possible to understand how the nanoparticles behave in the various metals, which are used as catalysts in the manufacturing process of industrial products. This knowledge is expected to contribute greatly to the creation of advanced and more efficient, environmental and cheaper catalysts in the various manufacturing industries.
