These syringes, which also contain nanotechnology, reduce the risk of infections in patients treated at home
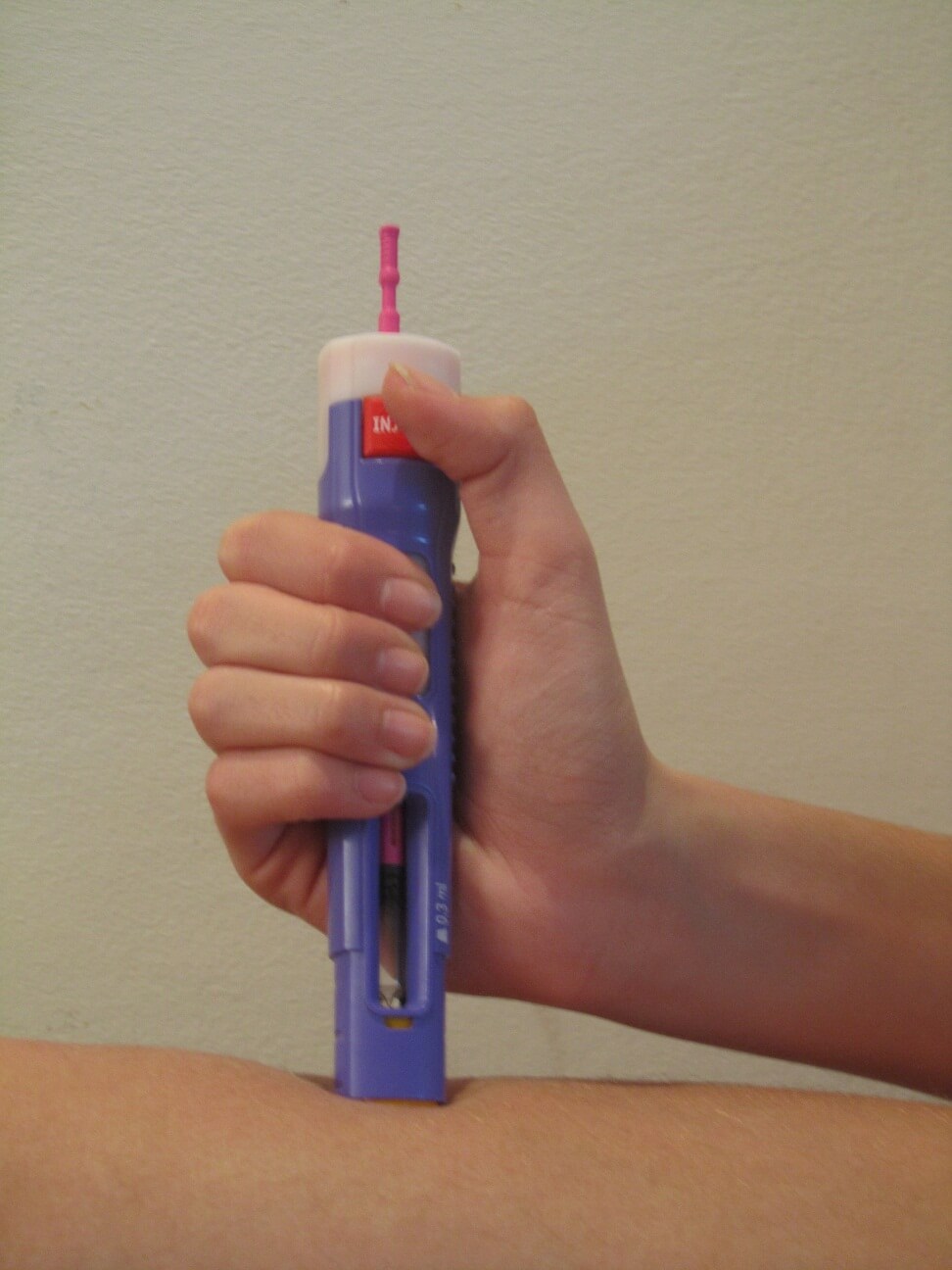
The Israeli company Elchem Medical, which develops and manufactures consumable medical products, will present for the first time at the ILSI-Biomed 2012 conference and exhibition, which will be held in Tel Aviv between May 21-23, the Flexi-Q family, which includes automatic, disposable syringes designed for injecting biological drugs in home use. The new syringes, approved for clinical use and marketing at the commercial level, guarantee that the patient will be able to inject himself with confidence, while preventing contact with the needle and injecting the entire amount of the substance in the syringe.
To this day, patients with diseases such as rheumatoid arthritis, cancer of various types and dialysis patients, some of whom are also elderly, who had to inject themselves with new biological drugs (when the price of the injection can even reach the value of hundreds of dollars for a single injection), have often encountered a situation where the entire amount was injected, or did not penetrate to the correct depth. In addition, sometimes the injection was unsafe, visible in the normal syringe was exposed to infections. Beyond that, the patients had to go to the clinic or hospitals to receive the injection, a situation that made it difficult for the patients, increased the burden on the medical staff and increased the costs.
In Elchem Medical's automatic injectors, the needles are not exposed. The user does not see the needle during the injection, and a large 'window' in the syringe allows to see the stages of the injection's progress. The needle penetrates to the appropriate depth and only after that the medicine is transferred to the body tissues and finally when the injection is finished, a "click" is heard signaling the end of the injection to the user.
The development of biological drugs is currently focused on all serious diseases, especially cancers of the various types and autoimmune diseases. The currently accepted estimate in the global drug market is that within 5-10 years about 50% of the drugs on the market will be biological. These drugs are characterized by the fact that they are based on protein molecules (proteins) engineered by genetic engineering for specific needs in the treatment of the disease.
Two syringes in the Flexi-Q family have been approved for clinical use and marketing at the commercial level, with one of them recently receiving US FDA approval. The company is developing additional products, which are developed in accordance with market requirements, including a reusable electronic injector that allows for daily injection with the adjustment of the required dose and the required depth of penetration and other capabilities when the consumable part is small and convenient to use.
"In recent years, we have recognized that the large pharmaceutical companies are beginning to invest very significant amounts of their development budgets in the development of innovative biological drugs," says Dr. Menahem Zucker, VP of Elchem Medical. "It is already possible today to identify a large number of biological drugs in their "pipelines" and these drugs will require an automatic injector to inject these expensive drugs - by home injection."
Elchem Medical, located in Kibbutz Bar'am on the northern border, develops and manufactures consumable medical products for use in hospitals, especially in intensive care units and operating rooms, and sells its products all over the world using the OEM method. The CEO of Elchem-Medical Ehud Raiwitz adds that Elchem recently acquired the Italian company Lucomed, in order to expand the range of products and become a factor in the field of dialysis as well.

One response
Successfully!!!