One hundred and forty years after the discovery of the element helium, the lightest noble gas, and despite all the best efforts of chemists, still refuses to enter into any chemical bond. A chemist from the University of Warsaw calculated and found that it is possible to create two new compounds containing a helium-oxygen bond.
One hundred and forty years after the discovery of the element helium, the lightest noble gas, and despite all the best efforts of chemists, still refuses to enter into any chemical bond. Now, a glimmer of hope appeared in Poland when a chemist from the University of Warsaw calculated and found that it was possible to create two new compounds containing a helium-oxygen bond.
Being the smallest, most chemically inert and least electrically polarized of the 1 known chemical elements, helium has been a challenge to chemists for generations. Unfortunately, no researcher has so far been able to experimentally confirm the existence of two important forms predicted and labeled as HeBeO2 and HHeFXNUMX.
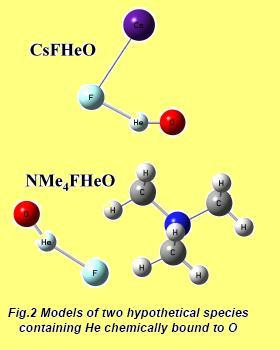
The two new molecular forms capable of binding helium to oxygen, predicted theoretically, and designated as NMe4FHeO and CsFHeO, are a consequence of the metastable anion [FHeO-] first predicted theoretically in 2005 by a group from Taiwan led by Professor Hu.
The scientist responsible for performing these new quantum chemical calculations is Dr. Wojciech Grochala from the Faculty of Chemistry at the University of Warsaw. The researcher points out: "The mules are not as strange as they could be at first glance. The idea is to preserve the metastable nature of the fine form [FHeO-] by linking it to a loose coordinating cation (such as Cs+ or NMe4+) in order to achieve electrical neutrality.
The resulting form contains a helium-oxygen bond with an electronic discharge energy of half an electron volt for the tetramethylammonium derivative. "Unfortunately, the kinetic stability of these compounds is extremely limited."
The practical implication of the search for these compounds indicates that at most they can be found at very low temperatures.
Referring to this goal, the researcher explains: "The preparation of the two forms can begin with the unusual fluorine ions of the form CsOF and NMe4OF, located inside a supercooled helium droplet. Excitation of the oxygen-fluorine chemical bond using a laser could allow the introduction of a helium atom into the interior of the bond and the spectroscopic observation of a suitable short-lived compound. Admittedly, experiments such as these are extremely challenging, but they are what make contemporary chemistry fascinating." The researcher adds and says: "Despite some difficulties, I am extremely excited about these predictions."
The news of the University of Warsaw

9 תגובות
Eyal, the fact that there may be two individual compounds of helium does not detract from its nobility. His outer shell is completely full, he was and will remain a noble gas, period.
And perhaps also the fact that now helium is no longer noble, its place is in the second column in the table, next to hydrogen, and not the last column belonging to the noble gases. If so, this is a very special thing and worthy of headlines in its own right.
Friends:
You are missing some important points here.
The simplest point is this:
When someone discovered the magnet - there must have been people who underestimated his words just as you underestimate the current article.
The more complex and important point:
Scientific research consists of an experiment, building theories and further experiments to check if the predictions of the theories are fulfilled.
Formulating a new prediction of an existing theory is therefore of enormous importance in that it creates another way to test the validity of the theory.
In the present case the scientist came and calculated a certain implication of the quantum theory.
If he was not mistaken in his calculations - something that will probably be checked - then he created here another way to confirm or refute the existing theory on the subject.
In general, I suggest that people here get rid of cynicism and really try to understand.
It's much more fun.
What can be done about it?
Where did all this theordorkis come from?
The paper buys everything. As long as the new compound is not created in a laboratory and its existence is not proven, then the argument must be taken with a limited guarantee. Looks more like an advertising gimmick (just like those "discoveries" of AIDS vaccines, etc.).
The term "Zuron" is the Hebrew terminology that refers to a part of an atom, molecule or a certain part of it, and is related to the chemical processes and reactions of that part.
Hanan Sabat
Following on from my daily talk
It really doesn't seem that apart from chemical curiosity, there is no breakthrough here.
There is a serious deterioration in the helium wells on the planet, so if it would help in any way to preserve the helium, no. I don't think it helps. Helium that is released into the atmosphere, disappears forever.
Shabbat Shalom
Sabdarmish Yehuda
I once studied chemistry...but is there anyone here who knows what "Tsuron" is?
I really didn't understand 95% of the article (I'm not a scientist, just someone who might be interested)
I understand that a breakthrough has been achieved here, but where to...?
How can this help us in our daily lives?