At very low temperatures, close to absolute zero, chemical reactions may occur at a much faster rate than expected by classical chemistry, because at these temperatures quantum phenomena come into play
At very low temperatures, close to absolute zero, chemical reactions may occur at a much faster rate than expected by classical chemistry, because at these temperatures quantum phenomena come into play. A team of scientists from the Weizmann Institute of Science verified this theoretical hypothesis experimentally. Their findings not only provide new insights into the quantum world where particles also behave as waves, but may also explain how chemical reactions occur in the frozen expanses of space.
Long-standing theories hold that at low temperatures, quantum phenomena cause the creation of a temporary chemical bond, which "forces" the colliding atoms and molecules to surround each other - instead of moving away (dispersing) from the collision site. Atoms and molecules in this type of bond have more opportunities to interact and carry out chemical reactions. According to this hypothesis, even a chemical reaction whose chances of occurring in the normal world are slim, can be carried out at a relatively fast pace, in the cold world where the temporary chemical bonds exist.
Dr. Ed Narevicius and his group members from the Department of Chemical Physics in the Faculty of Chemistry recently succeeded in performing a chemical reaction at a temperature of a hundredth of a degree above absolute zero (0.01°K - close to minus 273°C). Their findings were published this week in the scientific journal Science.
"In classical chemistry, we think of chemical reactions in terms of collisions between billiard balls, only on an atomic scale," says Dr. Narevičius. "According to this classical picture, energy reaction barriers prevent the spheres from approaching each other. In the world of quantum physics, on the other hand, the balls are able to pass through the barriers by tunneling, because at these ultra-low temperatures they acquire wave properties."
Efforts to observe quantum phenomena during chemical reactions began about half a century ago, with the groundbreaking experiments of Dudley Hershbach and Ewan T. Lee, who later won the Nobel Prize. They were able to observe chemical reactions resulting from the collision of two ultrasonic molecular beams. And yet, these collisions take place at high relative speeds, which are expressed in a reaction temperature above K°100 - too hot to allow quantum phenomena to occur. Since those pioneering experiments, scientists have used a variety of methods, including changing the angle of the beams and slowing their speed to a complete stop. Using these methods they managed to lower the temperature down to 5°K: closer to absolute zero, but still not cold enough to observe quantum phenomena.
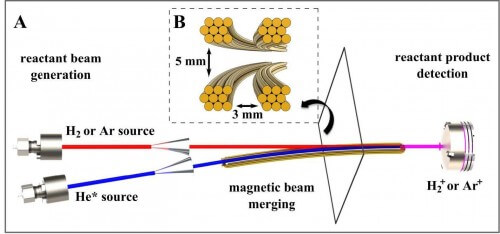
The innovation introduced by Dr. Narevičius and his team members, which included Alon Hanson, Sasha Gerstein, Yuval Shekh and Julia Narevičius, is a fusion of the two beams - instead of causing them to collide with each other. One beam was created and launched in a straight line, while the second beam was made to bend (using a magnetic field) until it moved parallel to the first beam. In this way, despite the high speed of the beams, the relative speed of the particles meeting each other was very low. This is how they managed to achieve a temperature of only a hundred degrees above absolute zero. One beam contained excited helium atoms, and the other beam contained argon atoms or hydrogen molecules. In the resulting chemical reaction, the argon atoms or hydrogen molecules were ionized, that is, they released electrons.
To test whether quantum phenomena were manifested during ionization, the scientists examined the rate of the reaction at different energies. The measurements showed that at high collision energies, the classical laws prevailed: the reaction rate decreased as the temperature decreased. But when the temperature dropped below 3°K, the results deviated from the expected classical predictions, and the reaction rate began to alternately increase and decrease. These measurements proved that at this stage a well-known quantum phenomenon of resonance following tunneling entered the picture: at low energies the particles begin to behave as waves, "leaking" through the energy barriers of the reaction, and undergo a constructive struggle with waves returned following the collision. At certain energies, standing waves are created in this way, which means that the particles are trapped for long periods of time in circular orbits around each other. Following this capture, the rate of the chemical reaction increased significantly.
"Our experiment is clear experimental proof that the rate of a chemical reaction can change dramatically at temperatures close to absolute zero," says Dr. Narevičius. "Beyond the surprising results we obtained, we proved that our method enables super-sensitive measurement of the dynamics of chemical reactions. Another thing that emerges from the findings is that our understanding of chemical reactions - even the simplest of them, such as ionization - is far from perfect. We must re-examine the existing theoretical models, and create better models. We expect that our method will be used to solve many mysteries involving chemical reactions, especially those that take place in space, which by their very nature do take place at extremely low temperatures."

One response
Totally cool.